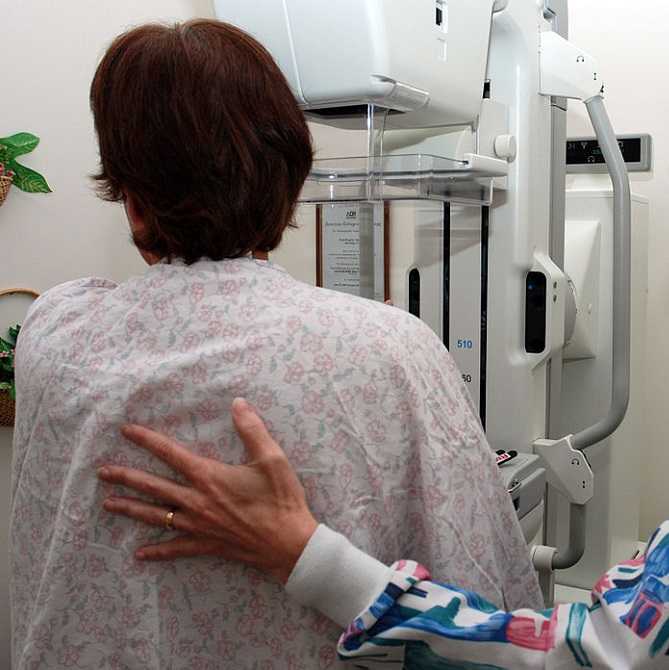
At a hair salon, I once saw a plaque that declared, “I’m a beautician, not a magician.” This crossed my mind while reading research on radical prostatectomy, as knowing the baseline penile function of men before surgery seemed challenging. Restoring something that may have been subpar prior to surgery can be a daunting task, and it can cause discrepancies in results of clinical trials. Despite this, two recent studies reviewed the current and future penile rehabilitation approaches post-radical prostatectomy.

Bratu et al.2017 published a review referring to post-radical prostatectomy (RP) erectile dysfunction (ED) as a challenge for patients as well as physicians. They emphasized the use of the International Index of Erectile Function (IIEF) Questionnaire to establish a man’s baseline erectile function, which can be affected by factors such as age, diabetes, alcohol use, smoking habits, heart and kidney diseases, and neurological disorders. The higher the IIEF score preoperatively, the higher the probability of recovering erectile function post-surgery. The experience of the surgeon and the technique used were also factors involved in ED. Radical prostatectomy is a trauma to the pelvis that negatively affects oxygenation of the corpora cavernosum, resulting in apoptosis and fibrotic changes in the tissue, leading to ED. Minimally invasive surgery allows a significantly lower rate of post-RP ED with robot assisted radical prostatectomy (RARP) versus open surgery. The cavernous neurovascular bundles get hypoxic and ischemic regardless of the technique used; therefore, the authors emphasized early post-op penile rehabilitation to prevent fibrosis of smooth muscle and to improve cavernous oxygenation for the potential return of satisfactory sexual function within 12-24 months.
Clavell-Hernandez and Wang2017 [and Bratu et al., (2017)] reported on various aspects of penile rehabilitation after radical prostatectomy. The treatment with the most research to support its efficacy and safety was oral phosphodiesterase type-5 inhibitors (PDE5Is), which help relax smooth muscle and promote erection on a cellular level. Sildenafil, vardenafil, avanafil, and tadalafil have been studied, either used on demand or nightly. Tadalafil had the longer half-life and was considered to have the greatest efficacy. Nightly versus on-demand for any PDE5I was variable in its results. Intracavernosal injection (ICI) and intraurethral therapy using alprostadil for vasodilation improved erectile function, but it caused urethral burning and penile pain. Vacuum erection devices (VED) promoted penile erection via negative pressure around the penis, bringing blood into the corpus cavernosum. There was no need for intact corporal nerve or nitric oxide pathways for proper function, and it allowed for multiple erections in a day. Intracavernous stem cell injections provided a promising approach for ED, and they may be combined with PDE5Is or low-energy shockwave therapy. Ultimately, the authors concluded early penile rehabilitation should involve a combination of available therapies.
Restoring vascularity to healing tissue is a primary goal in rehabilitation, and the sooner the better. Disruption of cavernous nerves and penile tissue post-RP demands rehabilitation, and some methods have more supporting clinical evidence than others. Newer approaches require more exposure and clinical trials for efficacy and long term outcomes. Clinicians should pay attention to updated research and consider taking continuing education courses such as Post-Prostatectomy Patient Rehabilitation or Oncology and the Male Pelvic Floor.
Bratu, O., Oprea, I., Marcu, D., Spinu, D., Niculae, A., Geavlete, B., & Mischianu, D. (2017). Erectile dysfunction post-radical prostatectomy – a challenge for both patient and physician. Journal of Medicine and Life, 10(1), 13–18.
Clavell-Hernández, J., & Wang, R. (2017). The controversy surrounding penile rehabilitation after radical prostatectomy. Translational Andrology and Urology, 6(1), 2–11. http://doi.org/10.21037/tau.2016.08.14
Lymphedema with regards to women’s health is most commonly associated with breast cancer. Upper extremity lymphedema can be limiting and painful without a doubt, and I have seen women suffering from irritating edema and limited shoulder range of motion and function after radical mastectomy. However, we may not always consider lower extremity lymphedema which can occur as a result of urogenital cancers and their treatments. Our knowledge and skilled hands can impact the quality of life of these patients who may seek treatment for their post-cancer complications.
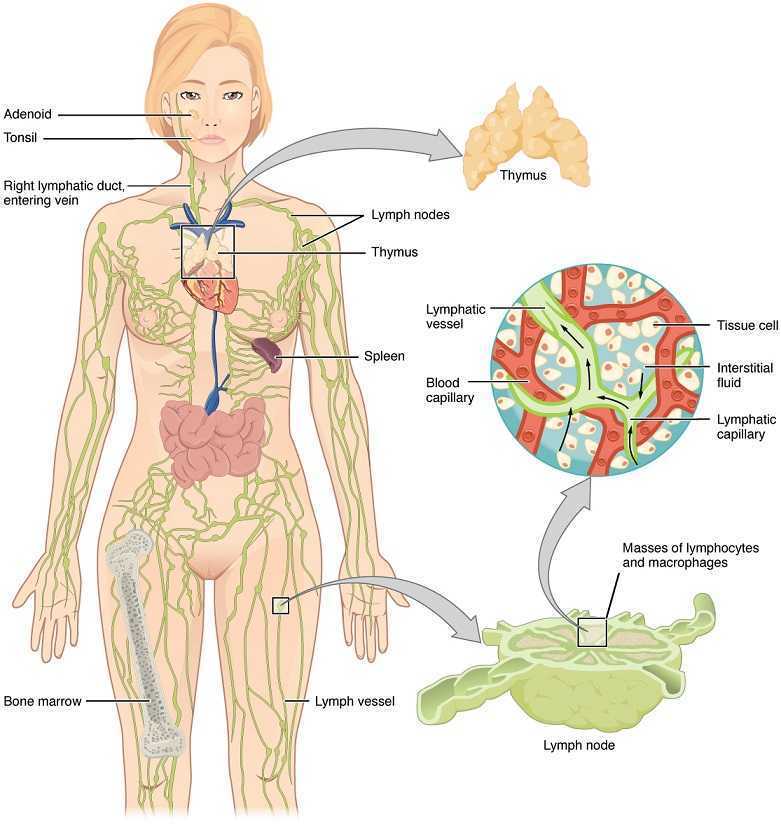 Mitra et al., published a 2016 retrospective study on lymphedema risk post radiation therapy in endometrial cancer. They considered 212 endometrial cancer survivors, and 7.1% who received adjuvant pelvic radiation therapy developed lower extremity lymphedema after treatment, whether they had chemotherapy or not. Finding at least 1 positive pathological lymph node was directly correlated with an increased risk of lymphedema, regardless of attempts to control pelvic lymph-node dissection. These statistics encourage finding prophylactic measures to take for stage III endometrial cancer patients to minimize the risk for long-term lymphedema. Regarding treatment for lower extremity lymphedema, this paper discussed compression stocking use, pneumatic compression stockings, and complex decongestive therapy, an intensive regimen of physical therapy and massage that is unfortunately not easily accessible for a majority of patients. The authors encouraged future research on the efficacy of exercise and compression for lymph node positive patients.
Mitra et al., published a 2016 retrospective study on lymphedema risk post radiation therapy in endometrial cancer. They considered 212 endometrial cancer survivors, and 7.1% who received adjuvant pelvic radiation therapy developed lower extremity lymphedema after treatment, whether they had chemotherapy or not. Finding at least 1 positive pathological lymph node was directly correlated with an increased risk of lymphedema, regardless of attempts to control pelvic lymph-node dissection. These statistics encourage finding prophylactic measures to take for stage III endometrial cancer patients to minimize the risk for long-term lymphedema. Regarding treatment for lower extremity lymphedema, this paper discussed compression stocking use, pneumatic compression stockings, and complex decongestive therapy, an intensive regimen of physical therapy and massage that is unfortunately not easily accessible for a majority of patients. The authors encouraged future research on the efficacy of exercise and compression for lymph node positive patients.
Shaitelman et al. presented a review of the progress made in the treatment and prevention of cancer-related lymphedema (2015). They stated gynecologic cancer treatment is associated with 25% incidence of lymphedema. Endometrial cancer had 1%, cervical cancer had 27%, and vulvar cancer had 30% incidence specifically. Sentinel lymph node biopsy (SLNB) can be an important part of cancer treatment, as lymphedema incidence was shown to average 9%. With treatment of genitourinary cancers, lymphedema occurred in 4% patients with prostate cancer, 16% patients with bladder cancer, and 21% patients with penile cancer. Shown to decrease limb volume and improve quality of life, the current standard of care is complete decongestive therapy (CDT). These authors state CDT involves the use of manual lymphatic drainage (MLD), bandaging on a daily basis, skin care, exercise, and a 3-phase protocol of compression. The use of SLNB helps identify the risk of lymphedema post cancer treatment; however, clinicians need to be aware of the signs and symptoms of lymphedema so the affected patient can be recognized early and referred to the appropriate specialist for treatment.
We may be referred patients with the confirmed diagnosis of lymphedema, but often we see post-cancer patients for rehab who develop lymphedema months after radiation or chemotherapy. Any healthcare professional involved with regular treatment of a cancer-surviving patient needs to have a keen sense for diagnosing and properly taking care of lymphedema. Courses such as “Lymphatics and Pelvic Pain: New Strategies” can open a whole new avenue for understanding what patients may need from us following urogenital and genitourinary cancers. Why not be prepared to face with knowledge and skill whatever pathology our patients present?
Mitra, D., Catalano, P.J., Cimbak, N., Damato, A.L., Muto, M.G., & Viswanathan, A.N. (2016). The Risk of Lymphedema After Postoperative Radiation Therapy in Endometrial Cancer. Journal of Gynecologic Oncology, 27(1), e4.
Shaitelman, S.F., Cromwell, K.D., Rasmussen, J.C., Stout, N.L., Armer, J.M., Lasinski, B.B., & Cormier, J.N. (2015). Recent Progress in Cancer-Related Lymphedema Treatment and Prevention. CA: A Cancer Journal for Clinicians, 65(1), 55-81.
A diagnosis of breast cancer means many different things to many different people. Regardless, receiving this diagnosis means some sort of treatment will likely follow. The types of treatment and outcomes are largely dependent on individual patient scenarios, however, one thing is for certain: A patient’s life will be forever changed after having received this diagnosis.
Historically, comprehensive care for a patient with breast cancer has focused on treatment and prevention. However, more and more women are surviving breast cancer every year. Therefore, more attention needs to be paid to survivorship. Once someone has survived cancer, comprehensive, quality care should obviously focus on preventing recurrence, however, it may also include guidance and counseling on maintaining a healthy lifestyle and addressing physical and psychosocial changes.
 A very recent 2016 article published in the Annals of Surgical Oncology discusses the subject of survivorship in breast cancer patients. This article suggests that the key to achieving successful outcomes for management of a breast cancer survivor is a multidisciplinary approach to help these survivors deal with the physical and psychosocial sequela resulting from their diagnosis.
A very recent 2016 article published in the Annals of Surgical Oncology discusses the subject of survivorship in breast cancer patients. This article suggests that the key to achieving successful outcomes for management of a breast cancer survivor is a multidisciplinary approach to help these survivors deal with the physical and psychosocial sequela resulting from their diagnosis.
As a pelvic rehabilitation provider, this is a very thought-provoking article as it outlines several areas in which I feel breast cancer survivors could benefit from physical therapy. A pelvic rehabilitation provider can be a valuable part of the multidisciplinary team that helps manage a breast cancer survivor towards positive and meaningful outcomes, ultimately enhancing their quality of life. The following are some areas addressed in the article in which a breast cancer survivor may need assistance to improve and support a meaningful quality of life.
Sexuality: According to this article, studies show treatment for breast cancer is associated with significant decrease in sexual interest, desire, arousal, and difficulty achieving orgasm and/or lack of sexual pleasure. Additionally, patients can also report pain with intercourse (dyspareunia) and/or vaginal dryness, which can lead to sexual dysfunction. Physical therapy can help by providing education on normal sexual response and lubricants, as well as help with tissue healing. Therapeutic techniques include exercise and manual treatments to areas that may be damaged from surgery, radiation, and chemotherapy. Additionally, exercise has been shown to improve self-image. Poor body image has been linked to sexual dysfunction following breast surgery (depending on the type “breast sparing techniques” versus mastectomy). This includes only some of the ways a physical therapist can help improve sexual dysfunction.
Lymphedema: According to the article, 30-70% of breast cancer patients experience lymphedema after treatment. Physical therapy can play an important role in the control and/or reduction of lymphedema. A physical therapist can provide helpful education, exercise, weight control, and, if needed, manual techniques and compression garments and bandaging.
Teachable moments after cancer diagnosis: A teachable moment is when you identify and seize an opportunity to educate your patient. After a life altering event or illness, people are more accepting of advice and change of lifestyle. As healthcare providers, we can utilize this time to help our patients improve outcomes by modifying their behavior. The cited article states there is clear evidence that physical activity decreases incidence and recurrence.There is additional evidence to show controlling weight and maintaining a normal body mass index (BMI) improves breast cancer survivor outcomes. A physical therapist can help a breast cancer survivor to develop a guided and progressive home exercise program to help them maintain normal BMI and participate in regular physical activity safely and regularly.
The discussed article, “Breast Cancer Survivorship: Why, What and When?”, sheds light on many areas of physical and psychosocial challenges that patients surviving breast cancer may deal with. This article also advocates that a multidisciplinary approach yields the greatest outcomes. I suggest that physical therapy can be a valuable part of the team when creating patient care plans for breast cancer survivors.
To learn more about breast cancer and outcomes based treatments, consider attending "Physical Therapy Treatment for the Breast Oncology Patient! The next course is taking place in Stockton, CA this September 24-25.
Gass, J., Dupree, B., Pruthi, S., Radford, D., Wapnir, I., Antoszewska, R., ... & Johnson, N. (2016). Breast Cancer Survivorship: Why, What and When?. Annals of Surgical Oncology, 1-6.
Susannah Haarmann, PT, CLT, WCS is the author and instructor of Physical Therapy Treatment for the Breast Oncology Patient. Join her this September 24-25 in Stockton, CA to learn about the various diagnostic tests, medical and surgical interventions to provide appropriate and optimal therapeutic interventions for breast cancer patients.
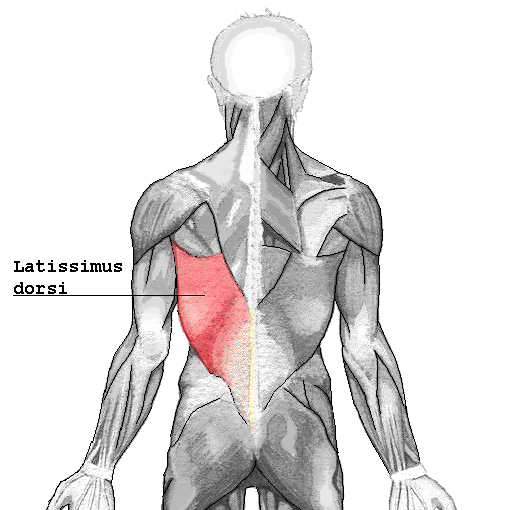 I turned to the literature and found prominent articles discussing breast reconstruction and giving minimal consequence to shoulder function after resection of the latissimus dorsi muscle. As a physical therapist, this left me in a quandary, “Really? Harvesting a portion of the broadest muscle of the back then threading it through the axilla to recreate the breast mound won’t have an impact on shoulder function or back pain? Impressive!” However, this did not correlate with my clinical findings. Often, scapulohumeral rhythm was altered, range of motion restricted and activities limited due to pain and fatigue. Scrutinizing the literature, I found that those articles were mostly unsubstantiated. Here is a quick summary of two systematic reviews published in 2014 addressing what the research really found pertaining to shoulder function after ‘lat flap’ reconstruction:
I turned to the literature and found prominent articles discussing breast reconstruction and giving minimal consequence to shoulder function after resection of the latissimus dorsi muscle. As a physical therapist, this left me in a quandary, “Really? Harvesting a portion of the broadest muscle of the back then threading it through the axilla to recreate the breast mound won’t have an impact on shoulder function or back pain? Impressive!” However, this did not correlate with my clinical findings. Often, scapulohumeral rhythm was altered, range of motion restricted and activities limited due to pain and fatigue. Scrutinizing the literature, I found that those articles were mostly unsubstantiated. Here is a quick summary of two systematic reviews published in 2014 addressing what the research really found pertaining to shoulder function after ‘lat flap’ reconstruction:
Patient impressions:
- Reported incidence of overall functional impairment is 41%. 8
- Overhead activities, lifting and pushing objects and high-level activities such as sport and housework were the most cumbersome. 1,7
- Subjective deficits did not resolve based on length of follow-up. 1
Strength:
- Greatest deficits are noted with reconstruction on the dominant side. 4
- Extension of the shoulder is the most common strength deficit followed by adduction then internal rotation. 8
- Objective strength deficits typically resolved within a year. 8,9
- Rehab should be ordered pro-actively. 4
Range of Motion:
- Active flexion is the most common restriction followed by abduction. 8
- Rarely were these restrictions severe. 5,6
- Restrictions were greatest post-operatively likely due to alterations in shoulder biomechanics, scar formation and post-operative pain.
- Discrepancies were found regarding resolution of range of motion without rehab. 5,8
- No clinically significant functional morbidity was found when therapy was provided from post-op day one. 2,3
Other reported complications that may impact function:
- Taratino, Banic and Fischer noted that capsular contracture was the most significant and recurrent complication in their study.10
- 50% reported post-operative numbness and tightness.1
- Scar tissue adhesions were associated with functional limitations.2,3
In conclusion, is it feasible to say that the latissimus dorsi muscle bears little consequence to function after reconstruction? I’m going to trust what the researchers performing the systematic reviews say:
- Physicians and researchers Lee and Mun state the following; “over 20 percent of the patients undergoing latissimus dorsi muscle transfer suffered from considerable disability…7% of patients changed their job postoperatively. These results suggest that considerable discomfort, even to the extent of limitation on daily activity, can be developed after latissimus dorsi muscle harvest, as opposed to the previous assumption that latissimus dorsi muscle harvest may not lead to serious disability” .8
- Smith does give merit to the fact that most strength deficits resolve within 6 to 12 months due to other muscles compensating for function, however, she states “standardization of physical therapy protocols is imperative as it appears to have a measurable positive impact.” Immediately after this statement she remarks that physical therapy is rarely included in the physician’s plan of care.9
I guess it is time we start talking to our surgical oncologists and plastic surgeons.
1. Adams, Jr., W., Lipschitz, A., Ansari, M., Kenkel, J., & Rohrich, R. J. (2004). Functional donor site morbidity following LD muscle flap transfer. Annals of Plastic Surgery, 53(1), 6–11.
2. de Oliveira, R., Nascimento, S., Derchain, S. & Sarian, L. (2013). Immediate breast reconstruction with a latissimus dorsi flap has no detrimental effects on shoulder motion or postsurgical complications up to 1 year after surgery. Plas¬tic and Reconstructive Surgery, 131(5), 673e–680e.
3. de Oliveira, R. R., Pinto e Silva, M. P., Costa Gurgel, M. S., Pas¬tori-Filho, L., & Sarian, L. O. (2010). Immediate breast re¬construction with transverse latissimus dorsi flap does not affect the short-term recovery of shoulder range of motion after mastectomy. Annals of Plastic Surgery, 64(4), 402– 408.
4. Forthomme, B., Heymans, O., Jacquemin, D., Klinkenberg, S., Hoff¬mann, S., Grandjean, F. X.,...Croisier, J. L. (2010). Shoulder function after latissimus dorsi transfer in breast reconstruc-tion. Clinical Physiology and Functional Imaging, 30, 406– 412.
5. Giordano, S., Kääriäinen, M., Alavaikko, J., Kaistila, T. & Kuok¬kanen, H. (2011). Latissimus dorsi free flap harvesting may affect the shoulder joint in long run. Scandinavian Journal of Surgery, 100, 202–207.
6. Hamdi, M., Decorte, T., Demuynck, M., Defrene, B., Fredricks, A., VanMaele, G.,...Monstrey, S. (2008). Shoulder func¬tion after harvesting a thoracodorsal artery perforator flap. Plastic and Reconstructive Surgery, 122(4), 1111–1117.
7. Koh, C. E., & Morrison, W. A. (2009). Functional impairment af¬ter latissimus dorsi flap. Australian Journal of Surgery, 79, 42–47. http://dx.doi.org/10.1111/j.1445-2197.2008.04797.x
8. Lee, K.T., Mun, G.H., (2014).A systematic review of functional donor-site morbidity after latissimus dorsi muscle transfer, Plast. Reconstr. Surg. 134: 303.
9. Smith, S., (2014). Functional morbidity following latissimus dorsi flap breast reconstruction. J Adv Pract Oncol, 5, 181–187.
10. Tarantino, I., Banic, A., & Fischer, T. (2006). Evaluation of late results in breast reconstruction by latissimus dorsi flap and prosthesis implantation. Plastic and Reconstructive Surgery, 117(5), 1387–1394.
Spending the past 5 years watching a lot of Disney Junior and reading Dr. Seuss, professional journal reading is generally reserved for the sanctuary of the bathroom. When patients ask if I’ve heard of certain new procedures or therapies, I try to sound intelligent and make a mental note to run a PubMed search on the topic when I get home. Making the effort to stay on top of research, however, makes you a more confident and competent clinician for the information-hungry patient and encourages physicians to respect you when it comes to discussing their patients.
A 2016 article in Translational Andrology and Urology, Lin et al., explored rehabilitation of men post radical prostatectomy on a deeper level, trying to prove that brain-derived neurotrophic factor (BDNF) promotes nerve regeneration. In many radical prostatectomies, even when the nerve-sparing approach is used, there is injury to the cavernous nerves, which course along the posterolateral portion of the prostate. Cavernous nerve injury can cause erectile dysfunction in 60.8-93% of males postoperatively. The authors discussed Schwann cells as being vital for maintaining integrity and function of peripheral nerves like the cavernous nerve. They hypothesized that BDNF, a member of the neurotrophin family that supports neuron survival and prevents neuronal death, activates the JAK/STAT (Janus kinase /signal transducer and activator of transcription) pathway in Schwann cells, thus facilitating axonal regeneration via secretion of cytokines (IL-6 and OSM-M). Through scientific experiment on a cellular level (please refer to the article for the specific details), the authors were able to confirm their hypothesis. Schwann cells do, in fact, produce cytokines that contribute to the regeneration of cavernous nerves.
From a different cellular perspective, Haahr et al., (2016) performed an open-label clinical trial involving intracavernous injection of “autologous adipose-derived regenerative cells” (ADRCs) in males experiencing erectile dysfunction (ED) after radical prostatectomy. Current treatments with PDE-5 inhibitors do not give satisfactory results, so the authors performed a human phase 1, single-arm trial to further the research behind the use of adipose-derived stem cells for ED. Some limitations included the study was un-blinded and had no control group. Seventeen males who had ED after radical prostatectomy 5-18 months prior to the study were followed for 6 months post intracavernosal transplantation. The primary outcome was safety/tolerance of stem cell treatment, and the secondary was improvement of ED. The single intracavernosal injection of freshly isolated autologous adipose-derived cells resulted in 8 of 17 men regaining erectile function for intercourse; however, the men who were not continent did not regain erectile function. The end results showed the procedure was safe and well-tolerated. There was a significant improvement in scores for the International Index of Erectile Function-5 (IIEF-5), suggesting this therapy may be a promising one for ED after radical prostatectomy.
In the clinic, we need to treat our patients to the best of our ability. Taking the Post-Prostatectomy Patient Rehabilitation course is vital if even just one patient enters your office seeking treatment. Keeping up on research (even that which seems too full of forgotten science) and learning new manual techniques and exercises can help us rise as clinicians prepared to optimize patients’ function.
Lin, G., Zhang, H., Sun, F., Lu, Z., Reed-Maldonado, A., Lee, Y.-C., … Lue, T. F. (2016). Brain-derived neurotrophic factor promotes nerve regeneration by activating the JAK/STAT pathway in Schwann cells. Translational Andrology and Urology, 5(2), 167–175. http://doi.org/10.21037/tau.2016.02.03
Haahr, M. K., Jensen, C. H., Toyserkani, N. M., Andersen, D. C., Damkier, P., Sørensen, J. A., … Sheikh, S. P. (2016). Safety and Potential Effect of a Single Intracavernous Injection of Autologous Adipose-Derived Regenerative Cells in Patients with Erectile Dysfunction Following Radical Prostatectomy: An Open-Label Phase I Clinical Trial. EBioMedicine, 5, 204–210. http://doi.org/10.1016/j.ebiom.2016.01.024
The American Society of Clinical Oncology convened their 2016 annual meeting over the weekend, and several of the presentations suggest new methods of preventing breast cancer recurrence.
Extended Hormone Therapy Reduces Recurrence of Breast Cancer
 Breast cancer patients who are treated with aromatase inhibitor therapy are generally prescribed the the estrogen drugs for a five year course. A new study has suggested that by doubling the length of hormone therapy, the recurrence rate for breast cancer survivors drops by 34%. The study included 1,918 women who underwent five years of hormone therapy with the drug letrozole. After five years, half of the group switched to a placebo while the other half were given an additional five year treatment.
Breast cancer patients who are treated with aromatase inhibitor therapy are generally prescribed the the estrogen drugs for a five year course. A new study has suggested that by doubling the length of hormone therapy, the recurrence rate for breast cancer survivors drops by 34%. The study included 1,918 women who underwent five years of hormone therapy with the drug letrozole. After five years, half of the group switched to a placebo while the other half were given an additional five year treatment.
Drug Used to Treat Type 2 Diabetes May Increase Breast Cancer Survivability
The Univerisity of Pennsylvania School of Medicine has published results from two recent studies which document the effects of Metformin, a drug commonly used to treat type 2 diabetes, on breast cancer and endometrial hyperplasia. The study tracked outcomes for 1,215 patients who were diagnosed and surgically treated for breast cancer. Patients who began to use metformin after their diagnosis were found to have a 50% higher survivability rate than those who did not use metformin.
The timing of metformin use is extremely important when it comes to breast cancer survivability rates. The study also found that patients who used metformin prior to their diagnosis were more than twice as likely to die than those who never used the drug.
Research Suggests a "Mediterranian Diet" May Reduce Breast Cancer Recurrence
A study has indicated that a diet rich in vegetables, fish, and olive oil may decrease the odds of a breast cancer survivor experiencing a relapse or recurrence of their cancer. The study tracked 300 women with early-stage cancer and found that those who ate a normal diet were more likely to experience a breast cancer recurrence. The findings build upon previous research which indicated that a Mediterranean diet, and especially extra virgin olive oil, could reduce breast cancer risk by 68%.
Want to Learn More?
Susannah Haarmann, PT, CLT, WCS is the author and instructor of Physical Therapy Treatment for the Breast Oncology Patient, a course offered through the Herman & Wallace Institute. This continuing education course for medical practitioners offers a rehabilitation perspective for providers who work with oncology rehabilitation patients. Join Dr. Haarmann this in Stockton, CA on September 24-25 to learn evaluation and treatment techniques necessary to make an outpatient therapist an essential member of any oncology team.
1) Paul E. Goss, et al. J Clin Oncol 34, 2016 (suppl; abstr LBA1)
https://www.asco.org/about-asco/press-center/news-releases/ten-years-hormone-therapy-reduces-breast-cancer-recurrence
2) Yun Rose Li. University of Pennsylvania, American Society of Clinical Oncology Annual Meeting 2016
http://www.eurekalert.org/pub_releases/2016-06/uops-ddm060316.php
3) http://www.scienceworldreport.com/articles/41404/20160606/mediterranean-diet-prevent-breast-cancer-recurring.htm
My first experience treating a patient with shoulder pain and limitations post-mastectomy just happened to be a local doctor’s sister. Luckily, I did not know this until a few sessions into her therapy. Ultimately, even more than normal, this patient’s outcome was a make or break situation for a future referral source. Her incredible spirit and optimism made the prognosis an inevitably positive one. Whether or not I had manual therapy training was a moot point, according to current research; however, from my perspective, I would not have been as competent in treating her without it.
 In June 2015, De Groef et al. performed a review of literature to investigate the efficacy of physical therapy for upper extremity impairments after surgical intervention for breast cancer. Eighteen randomized controlled studies were chosen for review regarding the efficacy of passive mobilization, myofascial therapy, manual stretching, and/or exercise therapy after breast cancer treatment. In the studies reviewed, physical therapy began at least 6 weeks post-surgical intervention. Combining general exercise with stretching was confirmed effective on range of motion (ROM) by 2 studies. One study showed the effect of passive mobilization with massage was null for pain or impaired ROM. No study showed any effect of myofascial therapy, one poor quality study supported the use of passive mobilization alone, and one study showed no effect of stretching alone. Active exercises were found more effective than no therapy or simply education in five studies. Early intervention was found to be beneficial for shoulder ROM in 3 studies, but 4 other studies supported delayed exercise to promote wound healing longer. Ultimately, pain and impaired shoulder ROM after operative treatment for breast cancer have been treated effectively by a multifactorial approach of stretching and active exercise. The efficacy of passive mobilization, stretching, and myofascial therapy needs to be investigated with higher quality research in the future.
In June 2015, De Groef et al. performed a review of literature to investigate the efficacy of physical therapy for upper extremity impairments after surgical intervention for breast cancer. Eighteen randomized controlled studies were chosen for review regarding the efficacy of passive mobilization, myofascial therapy, manual stretching, and/or exercise therapy after breast cancer treatment. In the studies reviewed, physical therapy began at least 6 weeks post-surgical intervention. Combining general exercise with stretching was confirmed effective on range of motion (ROM) by 2 studies. One study showed the effect of passive mobilization with massage was null for pain or impaired ROM. No study showed any effect of myofascial therapy, one poor quality study supported the use of passive mobilization alone, and one study showed no effect of stretching alone. Active exercises were found more effective than no therapy or simply education in five studies. Early intervention was found to be beneficial for shoulder ROM in 3 studies, but 4 other studies supported delayed exercise to promote wound healing longer. Ultimately, pain and impaired shoulder ROM after operative treatment for breast cancer have been treated effectively by a multifactorial approach of stretching and active exercise. The efficacy of passive mobilization, stretching, and myofascial therapy needs to be investigated with higher quality research in the future.
Another review of literature in 2010 by McNeely et al. used 24 studies to analyze the effectiveness of exercise intervention for upper extremity impairments after breast cancer surgical intervention. Ten of the studies focused on early versus late intervention, and all supported the earlier implementation of post-surgical exercises for ROM; however, wound drain volume and duration were increased in the subjects engaged in earlier exercises. Fourteen studies showed structured exercise intervention improved shoulder ROM significantly in the post-op period, and a 6-month follow up continued to show improved upper extremity function. No lymphedema risk was noted in any of the studies.
As with many areas of physical therapy, better research is needed to support what we do. We often treat with success in the clinic despite lack of strength in the evidence-based realm. After implementing glenohumeral and scapulothoracic mobilizations, soft tissue work in the posterior cervical and scapular muscles (avoiding lymph nodes), stretching, progressive resisted strengthening, and a home program, my patient regained full range of motion and function of the affected shoulder after her mastectomy. In retrospect, I should have written up a case study on this patient to contribute to our profession. At least after my patient was discharged, the clinic where I worked received a healthy supply of future referrals from her sister because of the positive results achieved with therapy.
If you are interested in learning evaluation and treatment techniques which can benefit breast oncology patients, consider a Herman & Wallace Physical Therapy Treatment for the Breast Oncology Patient course in 2016.
De Groef A, Van Kampen M, Dieltjens E, Christiaens MR, Neven P, Geraerts I, Devoogdt N. (2015). Effectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: a systematic review. Archives of Physical Medicine and Rehabilitation. 96(6):1140-53. doi: 10.1016/j.apmr.2015.01.006. Epub 2015 Jan 13.
McNeely ML, Campbell K, Ospina M, Rowe BH, Dabbs K, Klassen TP, Mackey J, Courneya K. (2010). Exercise interventions for upper-limb dysfunction due to breast cancer treatment. The Cochrane Database System of Reviews. (6):CD005211. doi: 10.1002/14651858.CD005211.pub2.
Michelle Lyons is instructor of "Oncology and the Female Pelvic Floor: Female Reproductive and Gynecologic Cancers", among other Herman & Wallace courses. We thought you might like to hear her expert analysis of current research going on in the field of gynecologic oncology, and the benefits therapeutic yoga can have on patient rehabilitation. Take it away, Michelle!

More than 65,000 women are diagnosed with gynecologic cancers (vulvar, vaginal, cervical, ovarian, endometrial) in the United States each year (Sohl et al 2012). Treatment options for these women include surgery, chemotherapy, radiation and hormone therapy – all of which have the potential to have local, regional and global effects on a woman’s body. The pelvic rehab specialist is in a unique position to hugely improve quality of life issues for these women – dealing with issues directly associated with pelvic health (urinary, sexual and bowel function and dysfunction) as well as more global issues such as bone health, peripheral neuropathies and musculoskeletal dysfunctions.
Yoga has enormous potential as a therapeutic tool for gynecologic cancer survivors and as exercise prescription experts, we can add yoga as a multi-purpose tool to our skill-set.
Empirical research on therapeutic yoga has been ongoing for several decades, including several recent studies conducted with cancer patients and survivors. Although most of the research looking at the benefits of yoga for cancer survivors has been done in the context of breast and prostate cancers, we can safely extrapolate many of the benefits associated with oncology rehab yoga, including its immediately obvious ability to improve flexibility, strength, balance, but also the impact yoga can have on decreasing inflammation, improving sleep and raising quality of life scores in pelvic cancer survivors.
Recent papers by Dewhirst et al showed how moderate exercise can improve the efficacy of chemotherapy and radiation by decreasing tumour hypoxia – they also discovered that this may limit metastatic aggression.
We also know that exercise can be potent medicine when it comes to dealing with the effects of cancer treatments, especially fatigue, bone health and cardiovascular function, which may disrupt return to exercise (Kerry et al 2005). But pelvic cancer patients may face extra barriers when it comes to returning to exercise, such as pelvic pain and concerns about continence, as well as diminished flexibility, balance and strength. But as Blaney et al concluded in their 2013 paper ‘…however, the main barriers reported were those that had the potential to be alleviated by exercise.’ And in my opinion, this can be achieved by integrating yoga into our pelvic oncology rehab programs.
These recent and exciting research findings have encouraged me to add a therapeutic yoga lab session to my Oncology & the Pelvic Floor course, which I will be teaching in NY next month. This is the last chance to catch this course stateside this year so I hope you will join me in White Plains to explore the many ways we can make a serious impact on pelvic cancer survivorship (Bring your yoga mat!)
References:
Psychooncology. 2013 Jan;22(1):186-94.
Cancer survivors' exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: a questionnaire-survey. Blaney JM1, Lowe-Strong A, Rankin-Watt J, Campbell A, Gracey JH.
Annals of Behavioral Medicine
April 2005, Volume 29, Issue 2, pp 147-153
A Longitudinal Study of Exercise Barriers in Colorectal Cancer Survivors Participating in a Randomized Controlled Trial
Kerry S. Courneya Ph.D., Christine M. Friedenreich Ph.D., H. Arthur Quinney Ph.D., Anthony L. A. Fields M.D., Lee W. Jones Ph.D., Jeffrey K. H. Vallance M.A., Adrian S. Fairey M.Sc.
JNCI J Natl Canc
Allison S. Betof, Christopher D. Lascola, Douglas H. Weitzel, Chelsea D. Landon, Peter M. Scarbrough, Gayathri R. Devi, Gregory M. Palmer, Lee W. Jones, and Mark W. Dewhirst
Modulation of Murine Breast Tumor Vascularity, Hypoxia, and Chemotherapeutic Response by Exercise
Today we present Part II of Michelle Lyons' discussion on sex after gynecologic cancer. Michelle will be teaching a course on this topic in White Plains in August!
In Part One of this blog, I looked at the sexual health issues women face after gynecologic cancer. In Part Two, I want to explore different treatment options that we as pelvic rehab specialists can employ to help address the many implications of cancer and cancer treatment
Treatment for gynecologic cancers, including vulvar, vaginal, cervical, endometrial and ovarian cancers, may include surgery, radiation therapy, chemotherapy, and/or hormonal therapy. We know that any of these approaches can have an adverse effect on the pelvic floor, as well as systemic effects on a woman’s body. Issues can include pain, fibrosis, scar tissue adhesions, diminished flexibility, fatigue and feeling fatigued and unwell. The effects on body image should not be under-estimated either. In their paper ‘Sexual functioning among breast cancer, gynecologic cancer, and healthy women’, Anderson & Jochimsen explore how ‘…body-image disruption may be a prevalent problem for gynecologic cancer patients…more so than for breast cancer patients’. The judicious use of manual therapy and local and global exercise prescription may be excellent pathways for a women to re-integrate with her body.
Many women will have to learn to care for a new colostomy or how to catheterize a continent urostomy. A woman who has had a vulvectomy will need sensitive counselling to understand that she can still respond sexually. Patients who have had a vaginectomy with reconstruction as part of a pelvic exenteration will need extensive rehab to help them achieve successful sexual functioning. We as pelvic rehab practitioners are in a uniquely privileged position – not only can we ask the questions and discuss the options but we are licensed to be ‘hands on’ professionals, using our core skills of manual therapy, bespoke exercise advice and educating our patients about a range of issues from the correct usage of lubricants, dilators, sexual ergonomics and brain/pain science. I am in the habit of describing pelvic rehab as the best specialty in physical therapy but I think this is especially true when it comes to the junction of oncology and pelvic health. This is where we can integrate our knowledge of neuro-science, orthopaedics, the lymphatic system and pelvic health to deal with the effects of pelvic cancers and their treatment.
In Farmer et al’s 2014 paper, ‘Pain Reduces Sexual Motivation in Female But Not Male Mice’ , the authors found that ‘Pain from inflammation greatly reduced sexual motivation in female mice in heat -- but had no such effect on male mice’. Unfortunately ongoing pelvic pain is a common sequela of treatment for gynecologic cancers – reasons ranging from post-operative adhesions, post-radiation fibrosis or vaginal stenosis or genital lymphedema. It is also worth bearing in mind the ‘rare but real’ scenario of pudendal neuralgia following pelvic radiation, as discussed by Elahi in his 2013 article ‘Pudendal entrapment neuropathy: a rare complication of pelvic radiation therapy.’
The good news is that we have much to offer. Yang in 2012 (‘Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: A randomized controlled trial’) showed that pelvic rehab improved overall pelvic floor function, sexual functioning and QoL measures for gynecological cancer patients. Yang’s pelvic rehab group (administered by an experience physiotherapist) displayed statistically significant differences in physical function, pain, sexual worry, sexual activity, and sexual/vaginal function. Gynecological cancer and treatment procedures are potentially a fourfold assault: on sexual health, body image, sexual functioning, and fertility. Sexual morbidity is an undertreated problem in gynecological cancer survivorship that is known to occur early and to persist beyond the period of recovery (Reis et al 2010). We have a good and growing body of evidence that pelvic rehab, delivered by skilled therapists, has the potential to address each of these issues. And perhaps, most encouraging, here is Yang’s conclusion: ‘…‘Pelvic Floor Rehab is effective even in gynecological cancer survivors who need it most.’ (Yang 2012)
Interested in learning more about the role of pelvic rehab in gynecologic cancer survivorship? Join me in White Plains in August!
The following comes to us from Herman & Wallace faculty member Michelle Lyons. Michelle travels the world spreading the word about pelvic rehabilitation and the powerful benefits it can have on a patient's everyday life. Michelle will be teaching her newest course, "Oncology and the Female Pelvic Floor: Female Reproductive and Gynecologic Cancers" in White Plains, NY this August 14 - 15. Join her to learn more about evaluating and treating oncology patients.

According to the Scientific Network of Female Sexual Health and Cancer, ‘Sexuality is an experience that really is at the intersection of mind, body and relationship, and cancer treatment can impact all three of those elements”. Dr Sharon Bober of Dana Farber says ‘Part of the problem is that doctors are so focused on saving a cancer patient's life that they forget to discuss issues of sexual health. My sense is that it's not about physicians or health care providers not caring about your sexual health or thinking that it's unimportant, but that cancer is the emergency, and everything else seems to fall by the wayside".
If you harness the power of Google to look up female sexual dysfunction after gynaecologic cancer, you may see phrases like ‘Possible sexual side effects…’ or ‘Cancer treatment can cause physical changes that make having sex more difficult’ or even ‘cancer treatments may make intercourse painful’. To call these descriptions ‘understatements’ does not really do them justice.
For many women post-gynaecological cancer, resuming sexual function can be a multi-faceted problem. Issues can range from dealing with Cancer Related Fatigue and nausea, vomiting or diarrhea to physical changes in the size and shape of the vaginal canal. Cancer treatments can also cause hormone imbalances and tissue damage. Add to this issues with post-surgical/radiation adhesions, a disruption to the ability to produce lubrication, challenges to the musculo-skeletal systems within the hips and the pelvis as well as the onset of medically induced menopause….well you get the picture.
In a 2009 paper, ‘Interventions for sexuality after pelvic radiation therapy and gynaecological cancer’, Katz talks about the fact that ‘…very little attention has been paid to the sexual difficulties women experience after radiation to the sexual organs. There are a limited number of interventions for the woman who has been treated for gynaecological cancer with radiation. These focus on the provision of information and some specific suggestions related to treating vaginal dryness, the need for vaginal dilatation after radiation therapy, and management of fatigue. In ‘A systematic review of sexual concerns reported by gynaecological cancer survivors’ (Abbot Anderson 2012), the author points out that common concerns in the physical dimension were dyspareunia, changes in the vagina, and decreased sexual activity.
In the psychological dimension, common concerns were decreased libido, alterations in body image, and anxiety related to sexual performance. And in the social dimension, common concerns were difficulty maintaining previous sexual roles, emotional distancing from the partner, and perceived change in the partner's level of sexual interest.
The good news is that you can return to a normal sex life after surviving gynaecological cancer – particularly with the help of a skilled pelvic rehab provider.
In part 2 of this blog series, I will look at specific interventions in sexual rehab for the gynaecological cancer survivor. Interested in learning more about pelvic rehab and oncology? Join me in White Plains in August!
















































