One of my favorite things as the instructor of the Menopause Transitions course is when participants ask questions. Whether it is something about their own menopause journey or when a patient is struggling with symptoms, it thrills me to provide resources and clarity to help them make informed decisions.
The following are some of the questions that have come up in class or that have been brought to my attention via email after participants are back in the clinic:
Given the many benefits of hormone therapy, should every patient take it during or after perimenopause for the prevention of chronic disease?
This is an excellent question! Based on recommendations from The Menopause Society, hormone therapy is approved for the treatment of vasomotor symptoms, genitourinary symptoms, and the prevention of osteoporosis.
Hormones are often lauded as a benefit for reducing both heart disease and dementia. While it has shown some benefit for heart disease, it is not recommended as a preventative treatment. The same holds true for prevention of neurodegenerative disease. The research on the benefits of hormones in cardiovascular and neurodegenerative disease reductions is often looking a different outcomes.
Each study may use a different type of estrogen. An oral estrogen blend (Premarin), an estradiol patch, and an oral estradiol can all have different effects on the body. You simply cannot extrapolate data from one study to the next if the type of estrogen studied was different. In addition, some research shows no benefit. Based on the current data, hormones are not a slam dunk for the prevention of heart disease and dementia. More studies with the types of hormones that are currently being prescribed are needed before recommending them as prevention.
Hormone therapy is very effective for improving bone density. Osteoporosis is a painless process of bone loss. If a person is not experiencing hot flashes and is concerned about their risk for osteoporosis, they could opt for a DEXA scan and then make an informed decision with their provider regarding hormone therapy.
Is there a dose of estrogen that is more beneficial for treating osteoporosis?
In the 2021 position statement for the management of osteoporosis, the Menopause Society cites a study that shows improved bone density with increased dosage. Oral estradiol doses of .02mg, .05mg, and .075mg after 2 years of treatment correlated with an improvement in lumbar spine bone density of .4%, 2.3%, and 2.7% (Greenwald et.al, 2005). While improvement can be gained from a smaller dose, a higher dose does have more benefit. Once again, informed decision-making with a knowledgeable provider is needed.
What are your go-to resources for all things menopause?
Based on the answers to the two previous questions, I think you can see that The Menopause Society is the gold standard when it comes to all things menopause. The position statements are available on their website and can be accessed for free. This includes guidelines on hormones, non-hormonal treatments, genitourinary syndrome of menopause, and osteoporosis. They also have monthly practice pearls, which include many pertinent topics on current treatments and health concerns for the patient in the transition.
Another great resource is Jen Gutner’s The Vagenda. While complete articles require a subscription to her Substack, she does offer a free email version that shares information about many of the topics flying around in the social media sphere. Her opinions are not always popular, but they are always research-based.
A final resource would be Women Living Better. This website was started by women frustrated with their own perimenopause experience and has resources for patients wanting to know more about options for treatment and symptoms experienced during this time. The founders have also been responsible for some interesting research regarding a survey of 3200 women in perimenopause. This is also available on their website.
Keep in mind that there are many social media influencers with millions of followers who also offer information. They have YouTube channels, Instagram, and websites. Menopause seems to be everywhere! While it is extremely valuable to get the message out there, the conclusions offered are often oversimplified in the attempt to push a quick and easy narrative. If I have learned anything in my knowledge journey, it is that there is no one-size-fits-all answer. Treatment is very individualized based on health status, risk factors, and personal preferences. Nuance is the key when it comes to offering the best outcomes.
If you would like to learn more on this topic, then join me on April 26-27, 2025, in Menopause Transitions and Pelvic Rehabilitation to understand more about the physiological consequences to the body as hormones decline and how to assist our patients in lifestyle habits for successful aging.
References:
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause, 2020. 27(9): p. 976-992.
- Management of osteoporosis in postmenopausal women: the 2021 position statement of The North American Menopause Society. Menopause, 2021. 28(9): p. 973-997.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause, 2022. 29(7): p. 767-794.
- Greenwald, M.W., et al., Oral hormone therapy with 17beta-estradiol and 17beta-estradiol in combination with norethindrone acetate in the prevention of bone loss in early postmenopausal women: dose-dependent effects. Menopause, 2005. 12(6): p. 741-8.
- The Nonhormone Therapy Position Statement of The North American Menopause Society" Advisory, P., The 2023 nonhormone therapy position statement of The North American Menopause Society. Menopause, 2023. 30(6): p. 573-590.
AUTHOR BIO
Christine Stewart, PT, CMPT
 Christine Stewart, PT, CMPT (she/her) graduated from Kansas State University in 1992 and went on to pursue her master’s degree in physical therapy from the University of Kansas Medical Center, graduating in 1994. She began her career specializing in orthopedics and manual therapy, then became interested in women’s health after the birth of her second child.
Christine Stewart, PT, CMPT (she/her) graduated from Kansas State University in 1992 and went on to pursue her master’s degree in physical therapy from the University of Kansas Medical Center, graduating in 1994. She began her career specializing in orthopedics and manual therapy, then became interested in women’s health after the birth of her second child.
Christine developed her pelvic health practice in a local hospital with a focus on urinary incontinence and prolapse. She left the practice in 2010 to work at Olathe Health to further focus on pelvic rehabilitation for all genders and obtain her CMPT from the North American Institute of Manual Therapy. She completed Diane Lee’s Integrated Systems Model education series in 2018. Her passion is empowering patients through education and treatment options for the betterment of their health throughout their lifespan. She enjoys speaking to physicians and to community-based organizations on pelvic health physical therapy.

Menopause represents a transformative phase in a woman’s life, and as pelvic health practitioners, it’s an opportunity for us to make a significant impact. Rather than being the “beginning of the end,” menopause can usher in a new era of freedom and empowerment. Women spend a significant portion of their lives post-menopause, free from the limitations of periods, pain, and hormonal fluctuations, but only if we, as clinicians, provide the tools and education necessary to thrive during this transition.
Historically, women’s health during menopause has been overlooked. In the past century, limited life expectancy meant menopause healthcare and research were rarely prioritized. Combine that with the research gap in women’s health and well-documented medical misogyny, and one can see how generations of women’s health has been neglected. However, with advancements in healthcare and advocacy, that’s changing—and we need to lead the way in reframing menopause management, starting with conservative pelvic health approaches before defaulting to hormonal therapies.
The Overlooked Connection: Hormones, Voice, and Pelvic Floor Health
Among the myriad symptoms of menopause, the interplay between hormonal changes, the voice, and the pelvic floor often goes unnoticed. Hormonal shifts, especially decreasing estrogen levels, significantly impact voice quality, including reduced pitch range, vocal endurance, and an increase in vocal fatigue and dryness. These changes mirror similar phenomena in the pelvic floor, where decreased tissue elasticity, sarcopenia, and altered pressure management can result in incontinence, prolapse, or pain.
The voice is highly sensitive to endocrine changes throughout life. For example:
- Puberty: Testosterone lengthens and thickens the vocal folds, lowering pitch.
- Menstrual Cycle: Dysphonia premenstrualis, linked to inflammation and hormonal fluctuations, reduces vocal range and endurance.
- Pregnancy: Thickened vocal folds and decreased pitch are common due to hormonal and fluid changes.
During menopause, these shifts intensify. A study in Menopause revealed that 46% of postmenopausal women experience voice changes, with 33% reporting significant quality-of-life impacts, such as reduced confidence and professional standing.
Menopause, Hormone Therapy, and the Voice
Take puberty for example, when the presence of testosterone changes the dimensions of the male vocal tract. The vocal folds become thicker and longer and the larynx size increases, which changes the “fundamental frequency of the voice.” By contrast, premenstrual voice changes have also been noted, which is known as dysphonia premenstrualis and is characterized by a loss of ability to achieve high notes, as well as vocal fatigue and reduced vocal range. Some of these changes are driven by inflammation, mucosal dryness, and decreased mucosal secretions caused by progesterone. Other researchers have noted that cervical and laryngeal smears, taken during the premenstrual period phase consistent with progesterone peak, were indistinguishable.
Likewise, women going through pregnancy, and specific to the menopause discussion, experience unique voice changes as well. Vocal abnormalities noted in the literature include vocal fold thickening, lowered vocal pitch, vocal fatigue, reduced vocal range, and failure to reach higher notes. Though anecdotally I have also seen women struggle with mid-range notes rather than high-range notes in clinical practice, which underscores the importance of evaluating each patient case-by-case instead of making broad assumptions about voice and pelvic health during perimenopause and menopause.
Of additional concern is the change that HT can have on the vocal folds, which overall can be positive. HT can improve glandular secretions above and below the vocal folds, enhance mucosal viscosity, increase pitch range, capillary permeability, and overall better tissue oxygenation. Estrogen is also a well-known inflammatory mediator, which can help protect and prevent damage to the vocal folds. Additionally, sarcopenia is known to impact vocal fold shape, which could lead to vocal fold bowing, vocalis atrophy, and subsequent glottal fold closure impairment.
The Voice-Pelvic Floor Connection (V2PF)
The interconnectedness of the voice, respiratory diaphragm, and pelvic diaphragm provides a unique lens for menopause care. These three diaphragms share connective tissue and neuromuscular pathways, which influence pressure regulation essential for vocalization, continence, and core stability.
The V2PF Method offers a systems-based, trauma-informed approach to evaluating and addressing these connections. By focusing on:
- The orofacial/cervico-laryngeal diaphragm,
- The respiratory diaphragm, and
- The pelvic diaphragm,
Clinicians can help patients improve coordination, endurance, and strength across these systems. Techniques like musculoskeletal ultrasound imaging allow practitioners to assess and enhance pressure management strategies, leading to improved vocal and pelvic floor outcomes.
Empowering Women Through Education and Treatment
While hormone therapy (HT) can improve vocal function by increasing glandular secretions and reducing inflammation, among other benefits, it’s not a one-size-fits-all solution. Clinicians should consider HT as part of a comprehensive plan that includes conservative interventions such as manual therapy, exercise, and lifestyle modifications tailored to individual needs. Understanding the hormonal and structural changes of menopause empowers both practitioners and patients. With a holistic and interdisciplinary method like the V2PF approach, we can help women reclaim their voices—literally and metaphorically—during menopause.
Learn More
Join Dr. Garner's course The Voice and the Pelvic Floor at Herman and Wallace scheduled on March 8th and October 5th, 2025 to explore the V2PF method and its applications in pelvic health. Together, we can revolutionize care for women navigating menopause.
Want resources for patient education? Start here: https://youtube.com/playlist?list=PLssRl7MibHhHdVqWHIkrAp51PaYecA9d0&si=nptOLwRPbcpoJAi2
Struggling with vocal and perimenopause or menopause issues as a healthcare provider? Ginger provides first consults free at www.garnerpelvichealth.com
Resources:
- Afsah, O. Effects of hormonal changes on the human voice: a review. Egypt J Otolaryngol, 40, 22 (2024).
- Abitbol J, Abitbol P, Abitbol B. Sex hormones and the female voice. J Voice. 1999 Sep;13 (3): 424-46.
- D’haeseleer E, Depypere H, Van Lierde K, (2013) Comparison of speaking fundamental frequency between premenopausal women and postmenopausal women with and without hormone therapy. Folia Phoniatr Logop 65: 78–83
- McCarthy, M., Raval, A.P. The peri-menopause in a woman’s life: a systemic inflammatory phase that enables later neurodegenerative disease. J Neuroinflammation, 17, 317 (2020)
- Bensoussan T, et al. Voice as a predictor of health during menopause. Presented at: Annual Meeting of The Menopause Society; Sept. 10-14, 2024; Chicago.
- Schneider B, van Trotsenburg M, Hanke G, Bigenzahn W, Huber J. Voice impairment and menopause. Menopause. 2004 Mar-Apr; 11 (2): 151-8.
- Awan, S. N. (2006). The aging female voice: Acoustic and respiratory data. Clinical Linguistics & Phonetics, 20 (2–3), 171–180. https://doi-org.elon.idm.oclc.org/10.1080/02699200400026918
- https://www.nursinginpractice.com/clinical/womens-health/menopause-confidence-loss-and-the-voice/?utm_source=chatgpt.com
- https://hermanwallace.com/blog/trauma-and-the-voice-to-pelvic-floor-connection
AUTHOR BIO:
Dr. Ginger Garner PT, DPT, ATC-Ret

Ginger Garner, PT, DPT, ATC-Ret, is a board-certified specialist in lifestyle medicine and an orthopedic and pelvic health therapist with advanced training in MSK ultrasound, dry needling, visceral and fascial mobilization, integrative, and functional medicine, including yoga, Pilates, mindfulness, and hormone health. A UNC-Chapel Hill graduate, Dr. Garner is the author of multiple textbooks, book chapters, and articles. Based in Greensboro, NC, she owns Garner Pelvic Health, hosts The Vocal Pelvic Floor podcast, and serves in multiple leadership, advocacy, and policy roles at the state and federal levels. Her clinical work focuses on voice to pelvic floor trauma-informed care for complex conditions including endometriosis, perimenopause and menopause care, hypermobility syndrome, and hip dysplasia.
Visit Dr. Garner at her clinical practice, Garner Pelvic Health, Living Well Institute, www.integrativelifestylemed.com, and on Instagram and YouTube @drgingergarner.com.

One of the most bothersome and common symptoms experienced by patients going through the menopausal transition is hot flashes (Freedman 2015). Vasomotor symptoms can vary in intensity from mild to debilitating (Gold et al., 2000), and patients can suffer from a flushed face up to a full sweat with the removal of clothing and a brisk fan required for relief. Hot flashes can affect a patient’s focus, sleep, and activity tolerance. These pesky flashes are also associated with several medical disorders including heart disease, dementia, and osteoporosis (Biglia et al, 2017).
What causes hot flashes isn’t entirely known. A variety of factors can be at play including genetics, personal experience, cultural influences, and medications (Biglia et.al, 2017), however, one of the predominant factors contributing to these flushes is decreasing or fluctuating estrogen levels. Declining estrogen is linked with the KnDy (kisspeptin-neurokinin B-dynorphin neurons) located in the hypothalamus. These neurons project to the thermoneutral zone also located in the hypothalamus. This zone regulates the temperature in the body. As estrogen levels diminish, these neurons hypertrophy. This causes an increase in activity to the thermoneutral zone making the patient more sensitive to temperature changes (Rance et al, 2013). A small shift in temperature causes a greater physiological response triggering the hot flash.
In their 2022 position statement on hormone therapy, the North American Menopause Society recommends estrogen as one of the most effective treatments for this symptom. It is cited as a safe and effective option which many choose for relief. For some patients, this is not an option due to either personal choice or contraindications from their medical history.
An adjunct or alternative treatment for hot flashes is cognitive behavioral therapy (CBT). It has been proven as an effective diminisher of hot flashes and can be utilized by patients through this transition (The Non-Hormonal Position Statement of the North American Menopause Society 2023). In the book Living Well Through the Menopause, authors Hunter and Smith describe the importance of utilizing cognitive behavioral therapy as a tool for diminishing the intensity and bother of hot flashes.
One of the tools specifically mentioned in their recommendations is diaphragmatic breathing. This is a common skill that can be taught to patients by providers to help manage pain and urinary urgency symptoms. By tapping into the parasympathetic or “rest and digest” aspect of the autonomic nervous system, it facilitates the body to chill out and calm. This can also be recommended to patients in the menopausal transition as one method of hot flash management. Quieting the nervous system throughout the day can aid in stress management and decrease the intensity of hot flashes.
Another tool for management is self-care (Hunter and Smith 2021). Perimenopause can be a time of great stress for many. Busy work schedules, aging parents, and active teenagers can cause patients to forget about prioritizing time for themselves to reflect, recharge, and pause. Patients' lives are often constant caregiving and chaos. With this flurry of activity, the importance of their own health and well-being can be forgotten. Clinicians are integral in reminding patients that taking time for themselves will ensure they are capable of handling the circus of activities they are juggling. Giving the patient permission for self-care can be invaluable. Encouraging exercise, friendships, and taking a rest can help with stress management and in turn can help with sleep and the severity of symptoms (Hunter and Smith 2021).
Ahhhhh sleep, so often disrupted in this phase of life. Hot flashes certainly play a role with this as does stress. Both can play off the other. Educating our patients about the effects of alcohol, caffeine, and bright light bombardment before bed can help them on the road to better rest. Teaching meditation or diaphragmatic breathing before bed can also provide benefits (Hunter and Smith 2021). With better sleep comes less stress and with less stress comes reduced symptoms.
When patients experience vasomotor symptoms, the pelvic health provider has several tools in their toolbox to help with management. There are non-hormonal options out there that can make a difference. Clinicians can help patients navigate through the menopausal transition with tools for decreasing the intensity of symptoms and improving quality of life.
To learn more, sign up for Menopause Transitions and Pelvic Rehab scheduled for February 1-2, 2025. This course is an excellent opportunity to understand the physiological consequences to the body as hormones decline, in order to assist our patients in lifestyle habits for successful aging. Course topics include cardiovascular changes, metabolic syndrome, bone loss and sarcopenia, neurological changes (headache, brain fog, sleeplessness), Alzheimer’s risk, and urogenital changes. Symptoms and treatment options will also be discussed, including hormone replacement, non-hormonal options, dietary choices, and exercise considerations.
References:
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause, 2022. 29(7): p. 767-794.
- Biglia, N., et al., Vasomotor symptoms in menopause: a biomarker of cardiovascular disease risk and other chronic diseases? Climacteric, 2017. 20(4): p. 306-312.
- Freedman, R.R., Menopausal hot flashes: mechanisms, endocrinology, treatment. J Steroid Biochem Mol Biol, 2014. 142: p. 115-20.
- Gold, E.B., et al., Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol, 2000. 152(5): p. 463-73.
- Hunter, M.a.S., Melanie, Living Well Through the Menopause: An Evidenced Based Cognitive Behavioural Guide 2021, Great Britain: Robinson.
- Rance, N.E., et al., Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol, 2013. 34(3): p. 211-27.
- The Nonhormone Therapy Position Statement of The North American Menopause Society" Advisory, P., The 2023 nonhormone therapy position statement of The North American Menopause Society. Menopause, 2023. 30(6): p. 573-590.
Author Bio
Christine Stewart, PT, CMPT
 Christine Stewart, PT, CMPT (she/her) graduated from Kansas State University in 1992 and went on to pursue her master’s degree in physical therapy from the University of Kansas Medical Center graduating in 1994. She began her career specializing in orthopedics and manual therapy then became interested in women’s health after the birth of her second child.
Christine Stewart, PT, CMPT (she/her) graduated from Kansas State University in 1992 and went on to pursue her master’s degree in physical therapy from the University of Kansas Medical Center graduating in 1994. She began her career specializing in orthopedics and manual therapy then became interested in women’s health after the birth of her second child.
Christine developed her pelvic health practice in a local hospital with a focus on urinary incontinence and prolapse. She left the practice in 2010 to work at Olathe Health to further focus on pelvic rehabilitation for all genders and obtain her CMPT from the North American Institute of Manual Therapy. She completed Diane Lee’s Integrated Systems Model education series in 2018. Her passion is empowering patients through education and treatment options for the betterment of their health throughout their lifespan. She enjoys speaking to physicians and to community-based organizations on pelvic health physical therapy.

According to the International Menopause Society, “The purpose of the day is to raise awareness of menopause and the support options available for improving health and well-being.” While we have come a long way in bringing this issue into the spotlight, I still encounter patients on a daily basis who are unaware of what this transition can mean to their long-term health and quality of life. Some do not discuss their symptoms with their physician, and others, despite sharing their symptoms with their doctor, often leave the visit with “just live with it” as the recommended course of care due to fear of breast cancer (Barber and Charles 2023).
I have been teaching my menopause course, Menopause Transitions and Pelvic Rehab, for almost three years. A common question raised by participants during the course is whether discussing hormonal treatment options is outside the scope of pelvic rehabilitation. While I understand the concern, I do have some thoughts I would like you to consider. To elaborate, let me share a recent clinical experience I had when evaluating a patient for an orthopedic pathology.
This patient was a cis-female patient in her late 40s who presented to the clinic for mid-back pain. She had an extensive medical history of auto-immune conditions and work-related injury. She had been hospitalized due to her diagnosis and had lost a substantial amount of weight. As with any therapy evaluation, I asked how she was sleeping.
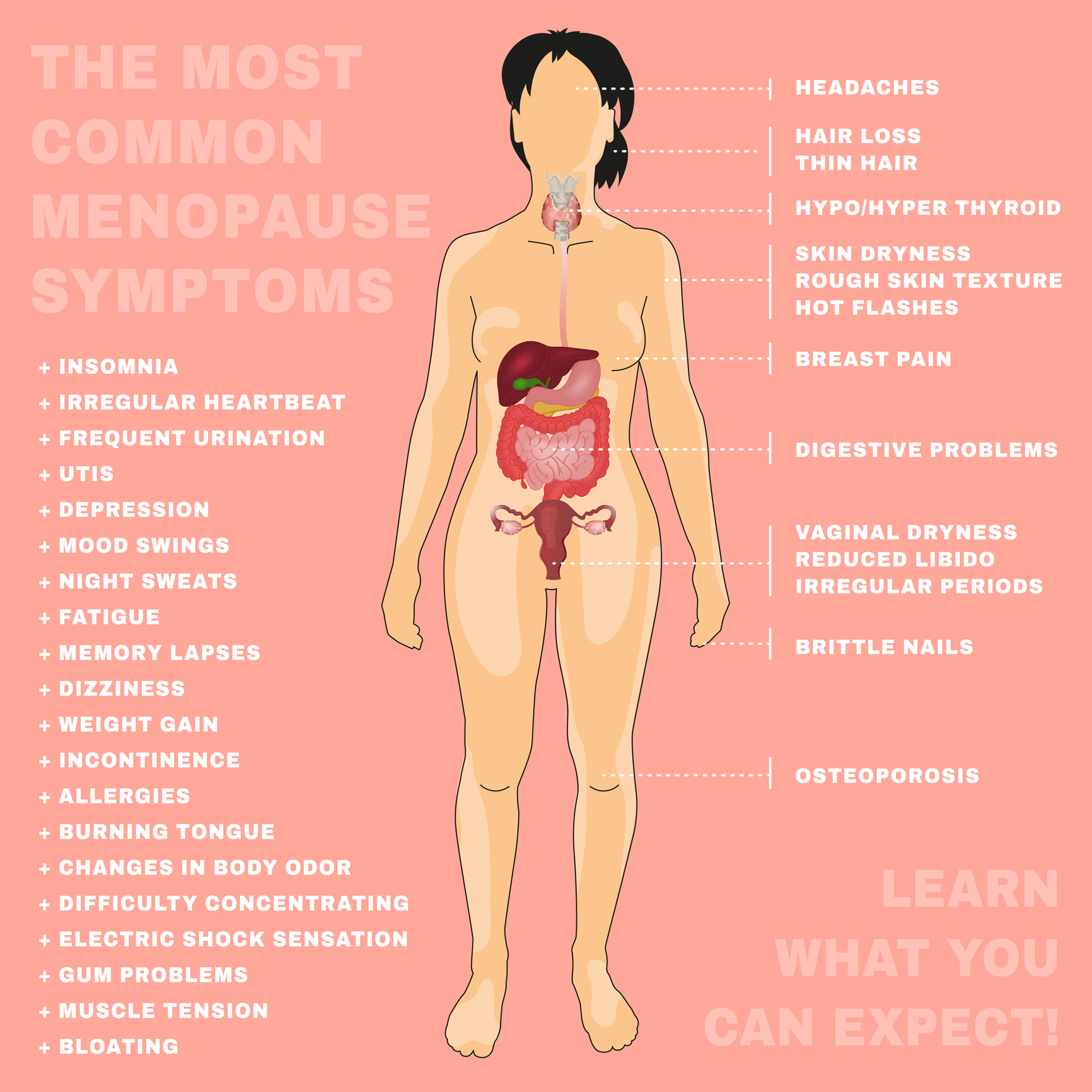 What she reported isn’t atypical for many of my patients. She was having pain that was disrupting her sleep patterns. She also reported hot flashes that were waking her up frequently in the night. She would go on walks occasionally but was not exercising regularly due to fatigue. Her food intake consisted of several servings of vegetables and fruit but was lacking in protein. On further discussion, she reported not having a period for a little over a year. She had misgivings about hormones due to breast cancer risk and wasn’t sure it was an option for her.
What she reported isn’t atypical for many of my patients. She was having pain that was disrupting her sleep patterns. She also reported hot flashes that were waking her up frequently in the night. She would go on walks occasionally but was not exercising regularly due to fatigue. Her food intake consisted of several servings of vegetables and fruit but was lacking in protein. On further discussion, she reported not having a period for a little over a year. She had misgivings about hormones due to breast cancer risk and wasn’t sure it was an option for her.
I was able to explain the background behind the Women’s Health Initiative and how this research was misinterpreted in the media and among many in the healthcare community regarding the safety of hormone therapy (Bluming and Tavris 2018). I explained estrogen is approved for the treatment of hot flashes and prevention of osteoporosis by The Menopause Society (position statement 2022). I also discussed the current recommendations of weight training from the Center for Disease Control of two days a week. We strategized goals for implementing this into her plan as she progressed in her exercise ability. I highlighted how the ability to process protein diminishes as she ages (Bauer et. al, 2013) requiring diligence to ensure adequate amounts in her diet.
On her next visit, she reported meeting with her obstetrics and gynecology doctor. She was given the option of hormone therapy and had started on a daily regimen. Her sleeping had improved, and she was feeling more like herself. She was making an effort to get protein at every meal, and while she was still not exercising consistently, she was hoping to start soon.
This patient was at high risk for osteoporosis due to her substantial weight loss and low BMI (Xiang et. al, 2017). Her sleep was being impacted by her hot flashes. Lack of sleep has been shown to increase the risk of cancer, heart disease, and neurodegenerative disease (Garborino et.al, 2021). It can also affect our patient’s ability to cope with stress and pain. How is someone supposed to heal if they can’t sleep? If I had not known about approved hormonal medications for the symptoms the patient was experiencing – I would not have been able to explain this option to the patient.
As pelvic health therapists, prescribing medications is certainly outside our scope. However, if we aren’t aware of their indications, how are our patients supposed to know their options? We are often the health care provider in whom they spend the most time and thus our patients often report symptoms to us that they haven’t shared with their doctor.
That is not to say that patients do not talk to their doctors about menopause. Many patients do actually report their symptoms to their physician; however, studies have found that these physicians often have not received the proper education on the menopause transition and are not always aware of when to prescribe hormone therapy (Kling et. al, 2019). We can provide referrals to specialists who DO have that knowledge so patients can know their options and make decisions together with an informed provider.
We can help to bridge the knowledge gap by knowing risks, benefits, and treatment options, not for prescription of medication, but as a conduit of information to give our patients options to discuss with their providers. This can allow for symptom management, improving quality of life, and in some instances prevention of fracture.
The more healthcare clinicians that have a basic understanding of menopausal symptoms and treatments, the more patients can be made aware of the options available to them. This allows for a dialogue to occur between physician and patient. Certainly, this is only a small piece of the treatment puzzle. There are many more important aspects of treatment including building muscle, sleep hygiene, stress management, and nutrition, which do fall within our scope.
In honor of World Menopause Day, let’s celebrate by raising awareness for our patients in regard to this change, and what can be done as they navigate through this transitional time. Join me on November 2-3, 2024 in Menopause Transitions and Pelvic Rehab to be a part of the conversation.
References:
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause, 2022. 29(7): p. 767-794.
- Barber, K. and A. Charles, Barriers to Accessing Effective Treatment and Support for Menopausal Symptoms: A Qualitative Study Capturing the Behaviors, Beliefs and Experiences of Key Stakeholders. Patient Prefer Adherence, 2023. 17: p. 2971-2980.
- Bauer, J., et al., Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc, 2013. 14(8): p. 542-59.
- Bluming, A.Z.T.C., Estrogen Matters. 2018, 1290 Avenue of the Americans, New York, NY 10104: Little, Brown Spark. 310.
- Garbarino, S., et al., Role of sleep deprivation in immune-related disease risk and outcomes. Commun Biol, 2021. 4(1): p. 1304.
- Kling, J.M., et al., Menopause Management Knowledge in Postgraduate Family Medicine, Internal Medicine, and Obstetrics and Gynecology Residents: A Cross-Sectional Survey. Mayo Clin Proc, 2019. 94(2): p. 242-253.
- Xiang, B.Y., et al., Body mass index and the risk of low bone mass-related fractures in women compared with men: A PRISMA-compliant meta-analysis of prospective cohort studies. Medicine (Baltimore), 2017. 96(12): p. e5290.
AUTHOR BIO
Christine Stewart, PT, CMPT
Christine Stewart, PT, CMPT (she/her) graduated from Kansas State University in 1992 and pursued her master’s degree in physical therapy from the University of Kansas Medical Center graduating in 1994. She began her career specializing in orthopedics and manual therapy then became interested in women’s health after the birth of her second child.
Christine developed her pelvic health practice in a local hospital with a focus on urinary incontinence and prolapse. She left the practice in 2010 to work at Olathe Health to further focus on pelvic rehabilitation for all genders and obtain her CMPT from the North American Institute of Manual Therapy. She completed Diane Lee’s Integrated Systems Model education series in 2018. Her passion is empowering patients through education and treatment options for the betterment of their health throughout their lifespan. She enjoys speaking to physicians and to community-based organizations on pelvic health physical therapy.

Tara Sullivan instructs her course Sexual Medicine in Pelvic Rehab on October 19-20. Her course provides a thorough introduction to pelvic floor sexual function, dysfunction, and treatment interventions of all sexual orientations, as well as an evidence-based perspective on the value of physical therapy interventions for patients with chronic pelvic pain related to sexual conditions, disorders, and multiple approaches for the treatment of sexual dysfunction including understanding medical diagnosis and management.
Menopause is a natural phase in a woman's life, signaling the end of her reproductive years. While many are familiar with common symptoms such as hot flashes, night sweats, brain fog, and mood changes, there is another less-discussed condition that affects many women: Genitourinary Syndrome of Menopause (GSM). GSM encompasses a range of symptoms affecting the genital and urinary systems, profoundly impacting a woman’s quality of life. Understanding GSM is crucial for women entering menopause and healthcare providers, especially pelvic floor specialists.
What is Genitourinary Syndrome of Menopause (GSM)?
Genitourinary Syndrome of Menopause (GSM) refers to a collection of signs and symptoms associated with the changes in estrogen levels that occur during menopause. These hormonal changes affect the tissues of the vulva, vagina, urethra, and bladder, leading to a variety of symptoms that can be both uncomfortable and disruptive.
GSM was formerly referred to as vulvovaginal atrophy, but this term was considered limited because it didn’t encompass the full scope of symptoms women experience, particularly those related to the urinary system. The term "GSM" is now preferred as it better reflects the diverse nature of the condition.
Common Symptoms of GSM
- Vaginal Dryness and Irritation: One of the most frequently reported symptoms of GSM is vaginal dryness. This occurs because estrogen levels drop, causing the vaginal tissue to become thinner, less elastic, and less lubricated. This dryness can lead to itching, burning, and irritation.
- Painful Intercourse (Dyspareunia): Vaginal dryness can make sexual activity uncomfortable or even painful. Women may also experience tearing or bleeding during intercourse due to the thinning of the tissue specifically around the vaginal opening.
- Urinary Symptoms: GSM can cause a range of urinary issues, including increased frequency of urination, urgency, urinary tract infections (UTIs), and incontinence. Estrogen plays a role in maintaining the health of the urinary tract, so its decline can lead to irritation and increased susceptibility to infections.
- Pelvic Floor Dysfunction: abnormal tone or weakening of pelvic floor muscles exacerbates urinary symptoms and pain, and contributes to conditions like pelvic organ prolapse.
- Changes in Vaginal pH: Estrogen plays a critical role in maintaining a healthy vaginal environment. With lower estrogen levels, the vaginal pH becomes less acidic, making the area more susceptible to infections such as bacterial vaginosis and yeast infections.
Causes and Risk Factors
GSM is directly related to the reduction in estrogen production during menopause. Estrogen is responsible for maintaining the thickness, elasticity, and moisture of the vaginal and urinary tissues. As levels drop, these tissues undergo changes that lead to GSM.
While GSM is most commonly associated with natural menopause, it can also occur in women who experience early menopause due to surgery or cancer treatments like chemotherapy and radiation. Women who smoke or have never given birth vaginally are also at a higher risk for developing GSM.
Treatment Options:
The good news is that GSM is treatable. While you might think, systemic hormone replacement therapy (HRT) is enough to resolve GSM, that’s not typically the case. More often, even if one is on already on estrogen HRT, or for those who cannot or will not take systemic estrogen, they can still apply a low dose estradiol cream specifically to the vestibule, urethra, and vaginal opening to target the tissue most affected by GSM. Local topical estradiol cream is considered a safe option. In a recent article, “In a large, claims-based analysis, we did not find an increased risk of breast cancer recurrence within 5 years in women with a personal history of breast cancer who were using vaginal estrogen for genitourinary syndrome of menopause”
However, if one is still opposed to using estradiol, other non-hormonal options are available to treat GSM symptoms:
- Vaginal Moisturizers and Lubricants: For women experiencing mild symptoms, over-the-counter vaginal moisturizers and lubricants can provide relief from dryness and discomfort. These products can be used regularly to help maintain vaginal moisture and make intercourse more comfortable.
- Pelvic Floor Physical Therapy: Many women with GSM benefit from pelvic floor physical therapy, which can strengthen the muscles of the pelvic floor, improve bladder control, and enhance sexual function. Physical therapists specialized in pelvic health can provide individualized treatments to address specific concerns.
- Laser Therapy: A newer, non-invasive option for GSM is laser therapy, such as fractional CO2 lasers. This therapy stimulates collagen production in the vaginal tissues, promoting healing and improving symptoms of dryness, pain, and laxity.
- Lifestyle Modifications: Quitting smoking, maintaining a healthy weight, and staying sexually active can also help reduce symptoms of GSM. Regular sexual activity increases blood flow to the vaginal area, helping to maintain tissue health.
Our Role as Pelvic Floor Therapist:
Despite affecting up to half of postmenopausal women, GSM remains underdiagnosed and undertreated. Many women may feel uncomfortable discussing these symptoms with their healthcare providers, or they may assume that these changes are a natural part of aging that must be endured. That is where pelvic floor specialists have a unique opportunity to educate these women. We have the luxury of one-on-one time and we are one of the only specialists that fully assess the vulvar tissue, specifically the vestibule and urethral opening where GSM is most identifiable. Understanding the research on estradiol treatment as well as other non-hormonal options can greatly improve our patients' quality of life.
Join Tara Sullivan in her upcoming course to learn more about Sexual Medicine in Pelvic Rehab on October 19-20. Lecture topics include hymen myths, squirting, G-spot, prostate gland, sexual response cycles, hormone influence on sexual function, anatomy and physiology of pelvic floor muscles in sexual arousal, orgasm, and function and specific dysfunction treated by physical therapy in detail including vaginismus, dyspareunia, erectile dysfunction, hard flaccid, prostatitis, post-prostatectomy; as well as recognizing medical conditions such as persistent genital arousal disorder (PGAD), hypoactive sexual desire disorder (HSDD) and dermatological conditions such as lichen sclerosis and lichen planus.
Resource:
- Agrawal P, Singh SM, Able C, Dumas K, Kohn J, Kohn TP, Clifton M. Safety of Vaginal Estrogen Therapy for Genitourinary Syndrome of Menopause in Women With a History of Breast Cancer. Obstet Gynecol. 2023 Sep 1;142(3):660-668. doi: 10.1097/AOG.0000000000005294. Epub 2023 Aug 3. PMID: 37535961. https://pubmed.ncbi.nlm.nih.gov/37535961/
AUTHOR BIO:
Tara Sullivan, PT, DPT, PRPC, WCS, IF
 Dr. Tara Sullivan, PT, PRPC, WCS, IF (she/her) started in the healthcare field as a massage therapist practicing for over ten years, including three years of teaching massage, anatomy, and physiology. During that time, she attended college at Oregon State University earning her Bachelor of Science degree in Exercise and Sport Science, and she continued to earn her Masters of Science in Human Movement and Doctorate in Physical Therapy from A.T. Still University. Dr. Tara has specialized in Pelvic Floor Dysfunction (PFD) treating bowel, bladder, sexual dysfunctions, and pelvic pain exclusively since 2012. She has earned her Pelvic Rehabilitation Practitioner Certification (PRPC) deeming her an expert in pelvic rehabilitation, treating men, women, and children. Dr. Sullivan is also a board-certified clinical specialist in women’s health (WCS) through the APTA and a Fellow of the International Society for the Study of Women's Sexual Health (IF).
Dr. Tara Sullivan, PT, PRPC, WCS, IF (she/her) started in the healthcare field as a massage therapist practicing for over ten years, including three years of teaching massage, anatomy, and physiology. During that time, she attended college at Oregon State University earning her Bachelor of Science degree in Exercise and Sport Science, and she continued to earn her Masters of Science in Human Movement and Doctorate in Physical Therapy from A.T. Still University. Dr. Tara has specialized in Pelvic Floor Dysfunction (PFD) treating bowel, bladder, sexual dysfunctions, and pelvic pain exclusively since 2012. She has earned her Pelvic Rehabilitation Practitioner Certification (PRPC) deeming her an expert in pelvic rehabilitation, treating men, women, and children. Dr. Sullivan is also a board-certified clinical specialist in women’s health (WCS) through the APTA and a Fellow of the International Society for the Study of Women's Sexual Health (IF).
Dr. Tara established the pelvic health program at HonorHealth in Scottsdale and expanded the practice to 12 locations across the valley. She continues treating patients with her hands-on individualized approach, taking the time to listen and educate them, empowering them to return to a healthy and improved quality of life. Dr. Tara has developed and taught several pelvic health courses and lectures at local universities in Arizona including Northern Arizona University, Franklin Pierce University, and Midwestern University. In 2019, she joined the faculty team at Herman and Wallace teaching continuing education courses for rehab therapists and other health care providers interested in the pelvic health specialty, including a course she authored-Sexual Medicine in Pelvic Rehab, and co-author of Pain Science for the Chronic Pelvic Pain Population. Dr. Tara is very passionate about creating awareness of Pelvic Floor Dysfunction and launched her website pelvicfloorspecialist.com to continue educating the public and other healthcare professionals.
In March 2024, Dr. Tara left HonorHealth and founded her company Mind to Body Healing (M2B) to continue spreading awareness on pelvic health, mentor other healthcare providers, and incorporate sexual counseling into her pelvic floor physical therapy practice. She has partnered with Co-Owner, Dr. Kylee Austin, PT.

Faculty member Christine Stewart, PT, CMPT began her career specializing in orthopedics and manual therapy and became interested in women’s health after the birth of her second child. Christine joined Olathe Health in 2010 to further focus on women’s health and obtain her CMPT from the North American Institute of Manual Therapy. She also went through Diane Lee's integrated systems model in 2018. Her course, Menopause Transitions and Pelvic Rehab is designed for the clinician that wants to understand the multitude of changes that are experienced in the menopause transition and how they affect the aging process.
Menopause. The M-word, the second puberty, is the final frontier of a hormonal roller coaster when there are twelve consecutive months with no menstruation. A time of celebration, right? No more cramps, hygiene products, menstrual cups, or moodiness – FREEDOM! Not so fast my fellow clinician!
The body goes through some serious, hormonal loop-the-loops leading up to the cessation of ovulation. Perimenopause is the stretch leading up to the final cycle and this stretch can feel like yoga on steroids. It can last TEN years, not including symptoms experienced after the transition takes place. Changes in cycle length, flow, anovulation, and yes, even ovulating twice are all stages of perimenopause. (Hale et al., 2009). These changes translate into symptoms: sleeplessness, brain fog, anxiety, palpitations, fatigue, painful intercourse, and joint stiffness are just a few things that can be experienced during this time (Lewis, 2021).
This transition can begin for patients during their mid-thirties, more commonly it begins during their forties, but eventually, all people that ovulate will experience it. For some, perimenopause can be much more challenging than after menopause. The perimenopause hormone guessing game begins. Some months, progesterone makes an appearance. The next month, mostly estrogen, and some months - neither are around very much at all. If there is an abrupt change in ovulation, such as with a complete hysterectomy, the symptoms will most likely be intensified due to the abrupt loss of hormones. (Gunter, 2020). Dealing with the changes of menopause can be challenging in a variety of ways (like a two-year-old wailing for a candy bar in the checkout line), but many things can help ease this transition.
With fluctuating hormones also comes changes to many systems in the body. Estrogen receptors are everywhere, and when hormone levels are changing, so does the body’s internal workings. Glucose metabolism, bone physiology, brain, and urogenital function are just some of the systems affected (Shifren et al., 2014). Perimenopause is not just a time of altered periods. It is also a critical time in a person’s health where an increased incidence of heart disease, diabetes, and bone loss can begin (Lewis 2021).
Preparing for menopause should be on our radar for patients in their twenties, thirties, and early forties before the process starts. Establishing healthy habits earlier instead of later can help for a more successful transition, however, it is never too late! Knowing the signs and symptoms of this phase can help us guide patients and ourselves to a better understanding of what is happening with the body in this adaptation. We can make recommendations on lifestyle, exercise, and meditation, as well as refer them to other knowledgeable providers when needed.
I have had countless patients sent to me for urinary frequency, incontinence, or painful intercourse who are in this transition, but no one has talked to them about what is happening to their bodies. You may be thinking to yourself, these patients have doctors. Why aren’t they getting the information from their physician? After all, these providers have had years of training. The reality is sometimes doctors do not receive the necessary education to treat menopausal patients.
In a survey of postgraduate trainees in internal medicine, family medicine, and obstetrics/gynecology, 90% felt unprepared to manage women experiencing menopause (Reid, 2021). Insert jaw drop here. As pelvic health providers, we can help to fill this knowledge gap and be a conduit to explaining the process. We can empower patients with education, treatments, and recommendations to flourish in this critical phase of life.
The menopause transition can be a time of great uncertainty. Not only are patients’ lives transforming as their children grow and their parents age, but their bodies are changing as well. We can ease their burden in this period of adaptation. By calming their fears through education, we can assure them that indeed, they are not losing their minds.
Knowledge is power, and I am all in when it comes to empowering patients. They can learn that menopause is a phase and does not define who they are as a person. It is possible to survive and come out on the other side still thriving, while learning how to cope during the process. There is hope!

Menopause Transitions and Pelvic Rehab is an excellent opportunity to understand the physiological consequences to the body as hormones decline, in order to assist our patients in lifestyle habits for successful aging. Lecture topics include cardiovascular changes, metabolic syndrome, bone loss and sarcopenia, neurological changes (headache, brain fog, sleeplessness), Alzheimer’s risk, urogenital changes, as well as symptoms and treatment options. These include hormone replacement, non-hormonal options, dietary choices, and exercise considerations.
Menopause Transitions and Pelvic Rehab course dates include April 9-10th and August 27-28th.A question that often comes up in conversation around menopause is that of pelvic health – the effects on bladder, bowel or sexual health…what works, what’s safe, what’s not? Is hormone therapy better, worse or the same in terms of efficacy when compared to pelvic rehab? Do we have a role here?
An awareness of pelvic health issues that arise at menopause was explored in Oskay’s 2005 paper ‘A study on urogenital complaints of postmenopausal women aged 50 and over’ stating ‘…Urinary incontinence and sexual problems, particularly decline in sexual desire, are widespread among postmenopausal women. Frequent urinary tract infections, obesity, chronic constipation and other chronic illnesses seem to be the predictors of UI.’
 Moller’s 2006 paper explored the link between LUTS (Lower Urinary Tract Symptoms) and sexual activity at midlife: the paper discussed how lower urinary tract symptoms (LUTS) have a profound impact on women’s physical, social, and sexual well being, and confirmed that LUTS are likely to affect sexual activity. However, they also found that conversely, sexual activity may affect the occurrence of LUTS – in their study a questionnaire was sent to 4,000 unselected women aged 40–60 years, and they found that compared to women having sexual relationship, a statistically significant 3 to 6 fold higher prevalence of LUTS was observed in women with no sexual relationship. They also found that women who ceased sexual relationship an increase in the de novo occurrence of most LUTS was observed, concluding that ‘…sexual inactivity may lead to LUTS and vice versa’.
Moller’s 2006 paper explored the link between LUTS (Lower Urinary Tract Symptoms) and sexual activity at midlife: the paper discussed how lower urinary tract symptoms (LUTS) have a profound impact on women’s physical, social, and sexual well being, and confirmed that LUTS are likely to affect sexual activity. However, they also found that conversely, sexual activity may affect the occurrence of LUTS – in their study a questionnaire was sent to 4,000 unselected women aged 40–60 years, and they found that compared to women having sexual relationship, a statistically significant 3 to 6 fold higher prevalence of LUTS was observed in women with no sexual relationship. They also found that women who ceased sexual relationship an increase in the de novo occurrence of most LUTS was observed, concluding that ‘…sexual inactivity may lead to LUTS and vice versa’.
So, who advises women going through menopause about issues such as sexual ergonomics, the use of lubricants or moisturisers, or provide a discussion about the benefits of local topical estrogen? As well as providing a skillset that includes orthopaedic assessment to rule out any musculo-skeletal influences that could be a driver for sexual dysfunction? That would be the pelvic rehab specialist clinician! Tosun et al asked the question ‘Do stages of menopause affect the outcomes of pelvic floor muscle training?’ and the answer in this and other papers was yes; with the research comparing pelvic rehab vs hormone therapy vs a combination approach of pelvic rehab and topical estrogen providing the best outcomes. Nygaard’s paper looked at the ‘Impact of menopausal status on the outcome of pelvic floor physiotherapy in women with urinary incontinence’ and concluded that : ‘…(both pre and postmenopausal women) benefit from motor learning strategies and adopt functional training to improve their urinary symptoms in similar ways, irrespective of hormonal status or HRT and BMI category’.
We must also factor in some of the other health concerns that pelvic health can impact at midlife for women – Brown et al asked the question ‘Urinary incontinence: does it increase risk for falls and fractures?’ They answered their question by concluding that ‘‘… urge incontinence was associated independently with an increased risk of falls and non-spine, nontraumatic fractures in older women. Urinary frequency, nocturia, and rushing to the bathroom to avoid urge incontinent episodes most likely increase the risk of falling, which then results in fractures. Early diagnosis and appropriate treatment of urge incontinence may decrease the risk of fracture.’
If you are interested in learning more about pelvic health, sexual function and bone health at Menopause, consider attending Menopause Rehabilitation and Symptom Management.
Sexual activity and lower urinary tract symptoms’ Møller LA1, Lose G. Int Urogynecol J Pelvic Floor Dysfunct. 2006 Jan;17(1):18-21. Epub 2005 Jul 29.
A study on urogenital complaints of postmenopausal women aged 50 and over. Oskay UY1, Beji NK, Yalcin O. Acta Obstet Gynecol Scand. 2005 Jan;84(1):72-8.
Do stages of menopause affect the outcomes of pelvic floor muscle training? Tosun ÖÇ1, Mutlu EK, Tosun G, Ergenoğlu AM, Yeniel AÖ, Malkoç M, Aşkar N, İtil İM. Menopause. 2015 Feb;22(2):175-84. doi: 10.1097/GME.0000000000000278.
‘Impact of menopausal status on the outcome of pelvic floor physiotherapy in women with urinary incontinence.’ Nygaard CC1, Betschart C, Hafez AA, Lewis E, Chasiotis I, Doumouchtsis SK. Int Urogynecol J. 2013 Dec;24(12):2071-6. doi: 10.1007/s00192-013-2179-7. Epub 2013 Jul 17
The new year is here and with it, lots of motivational posting about exercise and weight loss…but how is this desire for ‘new year, new you’ affecting peri-menopausal women with urinary dysfunction? It has been established that the lower urinary tract is sensitive to the effects of estrogen, sharing a common embryological origin with the female genital tract, the urogenital sinus. Urge urinary incontinence is more prevalent after the menopause, and the peak prevalence of stress incontinence occurs around the time of the menopause (Quinn et al 2009). Zhu et al looked at the risk factors for urinary incontinence in women and found that some of the main contributors include peri/post-menopausal status, constipation and central obesity (women's waist circumference, >/=80 cm) along with vaginal delivery/multiparity.
 Could weight loss directly impact urinary incontinence in menopausal women? In a word – yes. ‘Weight reduction is an effective treatment for overweight and obese women with UI. Weight loss of 5% to 10% has an efficacy similar to that of other nonsurgical treatments and should be considered a first line therapy for incontinence’ (Subak et al 2005) But do these benefits last? Again – yes! ‘Weight loss intervention reduced the frequency of stress incontinence episodes through 12 months and improved patient satisfaction with changes in incontinence through 18 months. Improving weight loss maintenance may provide longer term benefits for urinary incontinence.’ (Wing et al 2010)
Could weight loss directly impact urinary incontinence in menopausal women? In a word – yes. ‘Weight reduction is an effective treatment for overweight and obese women with UI. Weight loss of 5% to 10% has an efficacy similar to that of other nonsurgical treatments and should be considered a first line therapy for incontinence’ (Subak et al 2005) But do these benefits last? Again – yes! ‘Weight loss intervention reduced the frequency of stress incontinence episodes through 12 months and improved patient satisfaction with changes in incontinence through 18 months. Improving weight loss maintenance may provide longer term benefits for urinary incontinence.’ (Wing et al 2010)
The other major health issues facing women at midlife include an increased risk for cardiovascular disease, Type 2 Diabetes and Bone Health problems – all of which are responsive to lifestyle interventions, particularly exercise and stress management. In their paper looking at lifestyle weight loss interventions, Franz et al found that ‘…a weight loss of >5% appears necessary for beneficial effects on HbA1c, lipids, and blood pressure. Achieving this level of weight loss requires intense interventions, including energy restriction, regular physical activity, and frequent contact with health professionals’. 5% weight loss is the same amount of weight loss necessary to provide significant benefits for urinary incontinence at midlife.
Successful weight management depends on nutritional intake, exercise and psychosocial considerations such as stress management, but for the menopausal woman, hormonal balance can also have an effect on not only bladder and bowel dysfunction but changing metabolic rates, thyroid issues and altered weight distribution patterns. As pelvic rehab therapists, we are all aware that pelvic health issues can be a barrier to exercise participation but sensitive awareness of the other particular challenges facing midlife women can make the difference in developing a beneficial therapeutic alliance and a journey back to optimal health. If you would like to explore the topics surrounding optimal health at menopause, why not join me in California in February?
Climacteric. 2009 Apr;12(2):106-13. ‘The effects of hormones on urinary incontinence in postmenopausal women.’ Quinn SD, Domoney C. Menopause. 2009 Jul-Aug;16(4):831-6. The epidemiological study of women with urinary incontinence and risk factors for stress urinary incontinence in China’ Zhu L, Lang J, Liu C, Han S, Huang J, Li X. J Urol. 2005 Jul;174(1):190-5. Weight loss: a novel and effective treatment for urinary incontinence’ Subak LL, Whitcomb E, Shen H, Saxton J, Vittinghoff E, Brown JS. J Urol. 2010 Sep;184(3):1005-10. Effect of weight loss on urinary incontinence in overweight and obese women: results at 12 and 18 months Wing RR, West DS, Grady D, Creasman JM, Richter HE, Myers D, Burgio KL, Franklin F, Gorin AA, Vittinghoff E, Macer J, Kusek JW, Subak LL; Program to Reduce Incontinence by Diet and Exercise Group. J Acad Nutr Diet. 2015 Sep;115(9):1447-63. doi: 10.1016/j.jand.2015.02.031. Epub 2015 Apr 29. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lean, M, & Lara, J & O Hill, J (2007) Strategies for preventing obesity. In: Sattar, N & Lean, M (eds.) ABC of Obesity. Oxford, Blackwell Publishing.
Perimenopausal pelvic health issues are, for many of us, some of the most common issues that we see in the women that we work with. Urinary incontinence is one of the most important issues for peri- and postmenopausal women. In Melville’s study1 of U.S. women, half of the participants between the ages of 50 and 90 experienced urine leakage every month. Zhu’s 2008 study2 looked at the risk factors for SUI - Multiple vaginal deliveries, Age/postmenopausal status, Chronic pelvic pain, Obesity, lack of exercise, constipation, and hypertension. But what is not often (enough) looked at in the research, is the link between urinary dysfunction and sexual dysfunction – usually because questions aren’t asked or assumptions are made. In Mestre et al’s 2015 paper3, they write ‘…Integrating sexual health in clinical practice is important. In women with pelvic floor disorders, the evaluation of the anatomical defects, lower urinary tract function and the anorectal function often receives more attention than sexual function.’
 But are they linked?
But are they linked?
In Moller’s exploration of this topic, they report that lower urinary tract symptoms (LUTS) have a profound impact on women’s physical, social, and sexual wellbeing. Unsurprisingly (to pelvic rehab specialists at least!), they found that the LUTS are likely to affect sexual activity. Conversely, sexual activity may affect the occurrence of LUTS. The aims of the Moller study were to elucidate to which extent LUTS affect sexual function and to which extent sexual function affect LUTS in an unselected population of middle-aged women in 1 year. A questionnaire was sent to 4,000 unselected women aged 40–60 years. Compared to women having sexual relationship, a statistically significant 3 to 6 fold higher prevalence of LUTS was observed in women with no sexual relationship. In women who ceased sexual relationship an increase in the de novo occurrence of most LUTS was observed. In women who resumed sexual relationship a decrease in LUTS was observed. In women whose sexual activity was unchanged no change in the occurrence of LUTS. So they rightfully concluded ‘…sexual inactivity may lead to LUTS and vice versa.’
In my Menopause course, we will explore the range of perimenopausal pelvic health issues that many women face and their inter-related nature – not just with each other but also how orthopaedic, endocrine and gastro-intestinal health issues influence pelvic health and wellness. Interested in learning more? Come and join the conversation in California in February 2018!
1. Melville JL, et al. Urinary incontinence in US women: a population-based study. Arch Intern Med 2005;165(5):537-42 - See more at: http://www.nursingcenter.com/lnc/JournalArticle?Article_ID=698029#sthash.cm8A90tS.dpuf
2. Zhu L1, Lang J, Wang H, Han S, Huang J. Menopause. 2008 May-Jun;15(3):566-9. The prevalence of and potential risk factors for female urinary incontinence in Beijing, China
3. Mestre M, Lleberia J, Pubill J, Espuña-Pons M Actas Urol Esp. 2015 Apr;39(3):175-82. Epub 2014 Aug 28. Questionnaires in the assessment of sexual function in women with urinary incontinence and pelvic organ prolapse.
One of my greatest nemeses when I was racing at 30 years of age was a woman in her 50’s. Although I hated losing to her, I was always inspired by her speed at her age. She motivated me to continue training hard, realizing my fastest days could be yet to come. As I now race in the “master’s” category in my 40’s, I still find myself crossing the line behind an older competitor occasionally. Research shows I should take heart and keep in step with females who continue to move their bodies beyond menopause.
 Mazurek et al., (2017) studied how organized physical activity among post-menopausal women could reduce cardiovascular risk. The study included 35 sedentary women aged 64.7 ± 7.7 years who had no serious health issues. They all participated in the Active Leisure Time Programme (ALTP) 3 times per day for 40–75 minute sessions for 2 weeks, including 39 physical activities. Exercise intensity stayed within 40–60% of maximal HR, and ratings of perceived exertion (RPE) on the Borg scale stayed between 8 and 15 points. This exercise training was followed by 3 months of the Prevent Falls in the Elderly Programme (PFEP), which is a general fitness exercise program to prevent falls in the elderly. Health status was measured at baseline, 2 weeks into the program, and after 3 months. The results showed significant reductions in central obesity, which increased the exercise and aerobic capacity of the subjects and improved lipid profiles. A significant reduction also occurred in the absolute 10-year risk of death from cardiac complications. The authors concluded these exercise programs could be effective in preventing primary and secondary cardiovascular disease in the >55 years old female population.
Mazurek et al., (2017) studied how organized physical activity among post-menopausal women could reduce cardiovascular risk. The study included 35 sedentary women aged 64.7 ± 7.7 years who had no serious health issues. They all participated in the Active Leisure Time Programme (ALTP) 3 times per day for 40–75 minute sessions for 2 weeks, including 39 physical activities. Exercise intensity stayed within 40–60% of maximal HR, and ratings of perceived exertion (RPE) on the Borg scale stayed between 8 and 15 points. This exercise training was followed by 3 months of the Prevent Falls in the Elderly Programme (PFEP), which is a general fitness exercise program to prevent falls in the elderly. Health status was measured at baseline, 2 weeks into the program, and after 3 months. The results showed significant reductions in central obesity, which increased the exercise and aerobic capacity of the subjects and improved lipid profiles. A significant reduction also occurred in the absolute 10-year risk of death from cardiac complications. The authors concluded these exercise programs could be effective in preventing primary and secondary cardiovascular disease in the >55 years old female population.
Nyberg et al., (2016) took a physiological look at exercise training on the vascular function of pre- and postmenopausal women, studying the prostanoid system. Prostanoids are vasoconstrictors, and prostacyclins are vasodilators. The loss of estrogen in menopause affects the ability of the vasodilators to function properly or even be produced, thus contributing to vascular decline. The authors checked the vasodilator response to an intra-arterial fusion of a prostacyclin analog epoprostenol as well as acetylocholine in 20 premenopausal and 16 early postmenopausal women before and after a 12-week exercise program. Pre-exercise, the postmenopausal women had a reduced vasodilator response. The women also received infusion of ketorolac (an inhibitor of cyclooxygenase) along with acetylcholine, creating a vasoconstriction effect, and the vascular response was reduced in both groups. The infusions and analyses were performed again after 12 weeks of exercise training, and the exercise training increased the vasodilator response to epoprostenol and acetylcholine in the postmenopausal group. The reduced vasodilator response to epoprostenol prior to exercise in early postmenopausal women suggests hormonal changes affect the capacity of prostacyclin signaling; however, the prostanoid balance for pre and postmenopausal women was unchanged. Ultimately, the study showed exercise training can still have a positive effect on the vascularity of newly postmenopausal women.
There are randomized controlled clinical trials and scientific evidence supporting the importance to keep moving as women (and men) age. Menopause should not be a self-proclaimed pause from activity in life. Not everyone has to become a competitive athlete to preserve cardiac and vascular integrity as we age, but we need to engage in some physical activity to keep our systems running for years to come.
Those interested in learning more about menopause rehabilitation considerations should consider attending Menopause Rehabilitation and Symptom Management.
Mazurek, K., Żmijewski, P., Kozdroń, E., Fojt, A., Czajkowska, A., Szczypiorski, P., Tomasz Mazurek, T. (2017). Cardiovascular Risk Reduction in Sedentary Postmenopausal Women During Organised Physical Activity. Kardiologia Polska. 75, 5: 476–485. http://doi:10.5603/KP.a2017.0035
Nyberg, M., Egelund, J., Mandrup, C., Nielsen, M., Mogensen, A., Stallknecht, B., Bangsbo, J., Hellsten, Y. (2016). Early Postmenopausal Phase Is Associated With Reduced Prostacyclin-Induced Vasodilation That Is Reversed by Exercise Training: The Copenhagen Women Study. Hypertension. 68:1011-1020. https://doi.org/10.1161/HYPERTENSIONAHA.116.07866