September is Gynae Cancer Awareness Month – but how aware are we as clinicians of the signs and symptoms, the epidemiology and the sequalae of treatment afterwards? As pelvic rehab specialists, we have the privilege of helping women live well after cancer treatment ends, both on a ‘local’ pelvic area (bladder, bowel, sexual and pelvic pain management strategies) but also on a more ‘global’ level – dealing with issues such as cancer related fatigue, bone health and cardiovascular concerns.
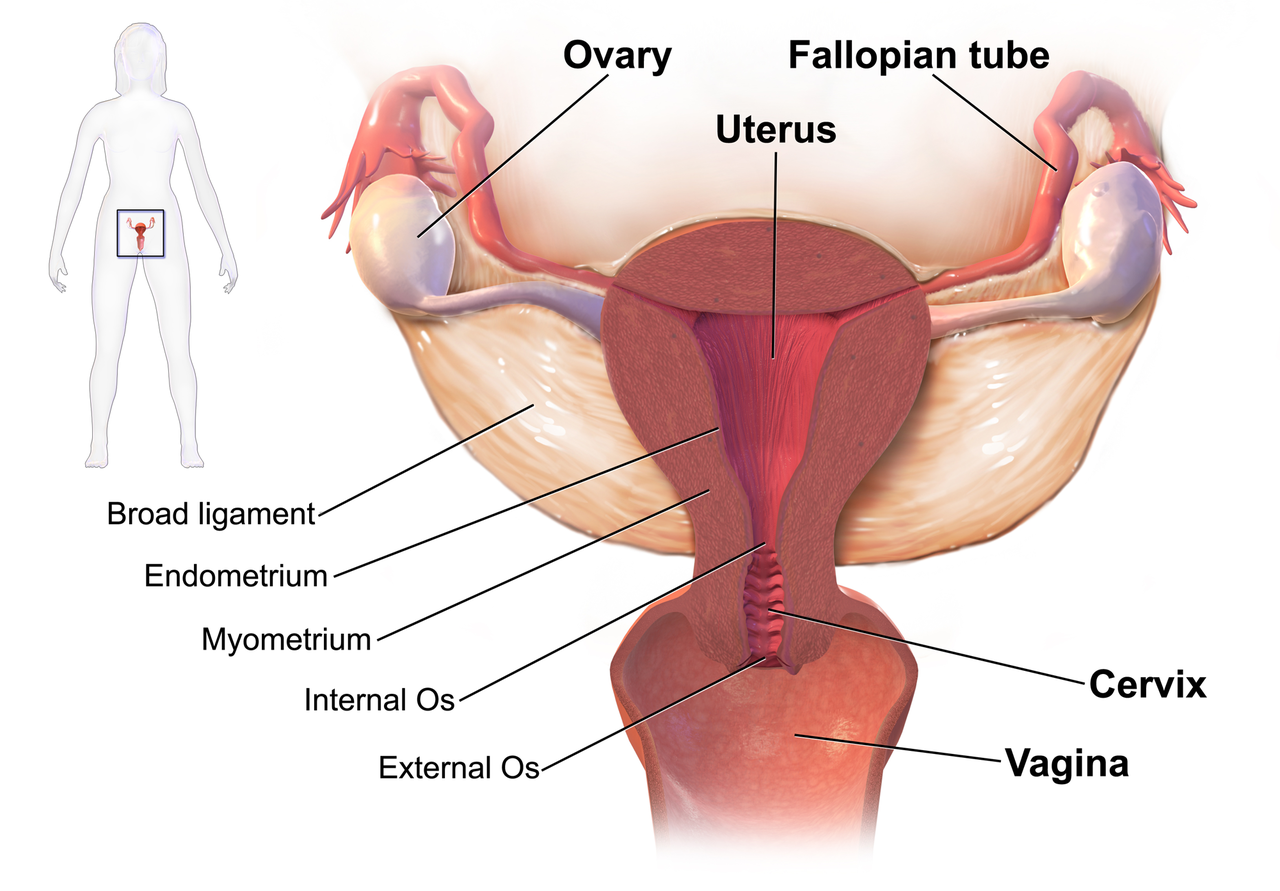 We know that women who are diagnosed with cancer of the vulva, vagina, cervix, endometrium or ovaries are treated with a combination of surgery, radiation or chemotherapy. However, with improving treatment and better survival rates, there is evidence that a variety of pelvic health concerns may arise for these women, both during and after treatment. (Hazewinkel et al 2010). For example, urinary incontinence is reported in 80% of women treated for endometrial cancer, with more severe symptoms and impact on quality of life in those who had adjuvant radiation (Erekson et al 2009) In Malone’s 2017 paper, ‘The patient’s voice: What are the views of women on living with pelvic floor problems following successful treatment for pelvic cancers?’, the author notes that ‘…there is currently a lack of knowledge regarding the effects of PFD on QoL in this cohort. Patients do not always report these problems to their health care providers and clinicians may underestimate symptoms…In the context of having survived cancer, PFD may be seen as relatively trivial. However, in the context of resuming normal living, the symptoms experienced by the survivors may be significant’.
We know that women who are diagnosed with cancer of the vulva, vagina, cervix, endometrium or ovaries are treated with a combination of surgery, radiation or chemotherapy. However, with improving treatment and better survival rates, there is evidence that a variety of pelvic health concerns may arise for these women, both during and after treatment. (Hazewinkel et al 2010). For example, urinary incontinence is reported in 80% of women treated for endometrial cancer, with more severe symptoms and impact on quality of life in those who had adjuvant radiation (Erekson et al 2009) In Malone’s 2017 paper, ‘The patient’s voice: What are the views of women on living with pelvic floor problems following successful treatment for pelvic cancers?’, the author notes that ‘…there is currently a lack of knowledge regarding the effects of PFD on QoL in this cohort. Patients do not always report these problems to their health care providers and clinicians may underestimate symptoms…In the context of having survived cancer, PFD may be seen as relatively trivial. However, in the context of resuming normal living, the symptoms experienced by the survivors may be significant’.
This can present a clinical conundrum – often pelvic rehab therapists are nervous when working with a patient who has a current or previous gynecologic cancer diagnosis, but similarly oncology rehab specialists may have qualms about dealing with pelvic health issues, with the result that these women fall through the cracks and do not have their pelvic health issues managed properly (or at all). Theodore Roosevelt once said ‘No one cares how much you know, until they know how much you care’ and this is especially relevant for oncology pelvic rehab. Often you may be the first clinician to ask about bladder, bowel or sexual function or dysfunction. An understanding of the effects of cancer treatments on the pelvis is important but so too is the wealth of information you may already have about bladder, bowel and sexual health as well as neuroscience and pain education.
The most important thing is to ask these women about their pelvic health concerns – the National Coalition for Cancer Survivorship defined cancer survivorship as extending from ‘the time of diagnosis and for the balance of life’. An emphasis on quality of life has been emphasised – if we know that cancer survivors may not independently volunteer information about their pelvic floor dysfunction, it is our responsibility to ask the questions and comprehensively treat and advocate for these women, in order to help them live well after cancer treatment ends.
- Hazewinkel MH. Sprangers MA, Velden Jvd, Vaart CH, Stalpers LJ, Burger MP ‘Longterm cervical cancer survivors suffer from pelvic floor dysfunction symptoms: A cross-sectional matched cohort study’ Gynecol Oncol 2010;117(2):381-6
- Erekson EA, Sung VW, Disilvestro PA, Myers DL ‘Urinary symptoms and impact on quality of life in women after treatment for endometrial cancer’ Int Urogynecol J 2009;20(2):159-63
- Malone P, Danaher D, Galvin R, Cusack T ‘The patient’s voice: What are the views of women on living with pelvic floor problems following successful treatment for pelvic cancers?’ Physiotherapy Practice and Research 38(2017)93-102
As so many of our patients are shallow breathers, I found this research on the effects of mindful attention to the breath (MATB) on prefrontal cortical and amygdala activity especially informative and relevant to patient care. Twenty-six healthy volunteers with no prior meditation experience were introduced to MATB by an experienced meditation teacher and instructed to practice a 20-minute audio guided MATB meditation daily for 2 weeks.1 At the end of the 2-week training period, subjects underwent fMRI scanning while viewing distressing emotional images with MATB and with passive viewing (PV). Participants were shown aversive pictures or no pictures and were instructed to “Please focus your attention on your breath as you were instructed in the training” or “Please watch the picture without changing anything about your feelings.” Subjects indicated their current affect on a 7 point scale ranging from -3 (very negative) to +3 (very positive).
Breathing frequency significantly decreased during MATB compared to PV. Researchers controlled for this by including breathing frequency as a covariate in further behavioral and brain data analysis.
Analysis of affective ratings showed that participants felt significantly less negative affect when viewing distressing visual stimuli during MATB than PV. During negative visual stimuli, MATB significantly decreased bilateral amygdala activation compared to PV. Also, right amygdala activation decrease specifically correlated with successful emotional regulation. That is, those participants with greater reductions in right amygdala activation reported greater reductions in aversive emotions during the MATB. In addition, emotion-related functional connectivity increased between the prefrontal cortex and amygdala during the viewing of negative images and MATB.
It’s exciting to have some initial science behind the benefits of MATB. I teach all of my patients MATB and have found it rewarding to get feedback from participants in my courses about their integration of MATB into their own patient care. Patients with complex pain conditions can be challenging to treat, however sometimes a simple practice of taking 2 to 3 minutes prior to and/or at the end of a treatment to have a patient calmly focus on their breath with the mindful attitudes of acceptance, kindness and curiosity can help a person shift from tension and distress to calm and confidence. I look forward to presenting this and additional research on the impact of mindful meditation on brain structure and function in my upcoming course, Mindfulness-Based Pain Treatment, in Seattle, November 4 and 5. Hope to see you there!
1. Doll A, Holzel BK, Bratec SM, et al. Mindful attention to breath regulates emotions via increased amygdala-prefrontal cortex connectivity. Neuroimage. 2016;134:305-313.
In a previous post on The Pelvic Rehab Report Sagira Vora, PT, MPT, WCS, PRPC shared that "cognitive-behavioral therapy appears to play a significant role in improving sexual function in women". Today, in part three of her ongoing series on sex and pelvic health, Sagira explores how sexual pain affects sexual dysfunction in women.
 After having explored what allows for women to have pleasurable sexual experiences including pain-free sex and mind-blowing orgasms, we now turn towards our cohort that have pain with sex and intimacy. How does this group differ from women who do not have pain with sex? Are there some common factors with this group of women, and perhaps understanding these factors may help the pelvic floor therapist render more effective and successful treatment?
After having explored what allows for women to have pleasurable sexual experiences including pain-free sex and mind-blowing orgasms, we now turn towards our cohort that have pain with sex and intimacy. How does this group differ from women who do not have pain with sex? Are there some common factors with this group of women, and perhaps understanding these factors may help the pelvic floor therapist render more effective and successful treatment?
There are few studies exploring sexual arousal in women with sexual pain disorders. However, their findings are remarkable. Brauer and colleagues found that genital response, as measured by vaginal photoplethysmography and subjective reports, was found to be equal in women with sexual pain vs. women who did not have pain, when they were shown oral sex and intercourse movie clips. This and other studies have shown that genital response in women with dyspareunia is not impaired. Genital response in women with dyspareunia is however, effected by fear of pain. When Brauer and colleagues subjected women with dyspareunia to threat of electrical shock (not actual shock) while watching an erotic movie clip they found that women with dyspareunia had much diminished sexual response including diminished genital arousal. But Spano and Lamont found that genital response was diminished by fear of pain equally in women with sexual pain and women without sexual pain.
Fear of pain also resulted in increased muscle activity in the pelvic floor. However, this increase was noted in women with pain and women without sexual pain equally and was noted with exposure to sexually threatening film clips as well as threatening film clips without sexual content. The conclusion, then, from these results is that the pelvic floor plays a role in emotional processing and tightening, or overactivity is a protective response noted in all women regardless of sexual pain history.
The one difference that was noted was with women who had the experience of sexual abuse. For them, pelvic floor overactivity was noted when watching sexually threatening as well consensual sexual content. Women without sexual abuse history did not have increased pelvic floor activity when watching consensual sexual content.
In summary, evidence supports the hypothesis that women with sexually adverse experiences tend to have impaired genital response when in consensual sexual situations, however, women who do not have sexual abuse histories and but have sexual pain tend to have appropriate genital response. Both groups, however, have increased pelvic floor muscle activity in consensual sexual situations. This increase in pelvic floor muscle activity leads to muscle pain, reduced blood flow, reduced lubrication, increased friction between penis and vulvar skin and hence leads to pain.
This brings us to our next questions, how does the cohort that has had adverse sexual experiences present? How do women with history of sexual trauma process sexual experiences? How does the pelvic floor present or respond to consensual sexual situations when a woman has been abused in the past? Please tune in to the next blog for answers…
Blok BF, Holstege G. The neuronal control of micturition and its relation to the emotional motor system. Prog Brain Res. 1996; 107:113-26
Brauer M, Laan E, ter Kuile MM. Sexual arousal in women with superficial dyspareunia. Arch Sex Behav. 2006; 35:191-200
Brauer M, ter Kuile MM, Janssen S, Lann E. The effect of pain-related fear on sexual arousal in women with superficial dyspareunia. Eur J Pain: 2007; 11:788-98
Spano L, Lamont JA. Dyspareunia: a symptom of female sexual dysfunction. Can Nurse 1975;71:22-5
For many of our patients, chronic pain is a chronic stress. Unfortunately, the resulting ongoing physiological stress reaction can have neurotoxic influences in key brain regions, including the prefrontal cortex, amygdala and hippocampus, and drive maladaptive neuroplastic changes that may further fuel a chronic pain condition.1 For example, chronic stress generates extensive dendritic spine loss in the prefrontal cortex, hyperactivity in the amygdala, and neurogenesis suppression in the hippocampus.2,3,4 In parallel, patients with chronic pain have been shown to exhibit reduced gray matter in the prefrontal cortex, increased neuronal excitability in the amygdala and reduced hippocampal neurogenesis.5,6,7
These three brain areas have been identified to play an important role in fear learning and memory.8 Modulated by stress hormones and stress-induced neuroplastic changes, stress may:
(a) enhance the memory of the initial pain experience at pain onset
(b) promote the later persistence of the pain memory
(c) impair the memory extinction process and the ability to establish a new memory trace.9
 In other words, an ongoing stress reaction, triggered by distressing cognitions and emotions in response to pain or other life circumstances, could reinforce and strengthen the memory of pain. The experience of pain could be generated not by nociceptive activity, but by a well-established memory of pain and inability of the brain to create new associations. Leading researchers in the cortical dynamics of pain at Northwestern University suggest this learning process and persistence of pain memory could be a major influencing mechanism driving chronic pain.9,10
In other words, an ongoing stress reaction, triggered by distressing cognitions and emotions in response to pain or other life circumstances, could reinforce and strengthen the memory of pain. The experience of pain could be generated not by nociceptive activity, but by a well-established memory of pain and inability of the brain to create new associations. Leading researchers in the cortical dynamics of pain at Northwestern University suggest this learning process and persistence of pain memory could be a major influencing mechanism driving chronic pain.9,10
In addition, neurogenesis suppression in the hippocampus is associated with depression, while increased amygdala excitability is associated with anxiety, two mood disorders that frequently accompany and complicate chronic pain conditions.11,12
Why is this important? Appreciating the complex factors that contribute to chronic pain conditions can point to treatment strategies that address these factors.13 For example, strategies that help reduce a patient’s stress reaction, mitigate the experience of fear and anxiety, and/or promote relaxation, positive mood and self-efficacy could conceivably reduce the stress reaction and reverse maladaptive neuroplasticity. While chronic pain is a multifaceted and highly complex condition with no simple answers or one-size-fits-all successful treatment strategy, initial research suggests promise for this approach to modulate cortical structure. In a study of cognitive-behavioral therapy (CBT) in the treatment of chronic pain, an 11-week CBT treatment course increased gray matter in the prefrontal cortex and hippocampus.14
In addition, a systematic review of brain changes in adults who participated in Mindfulness-Based Stress Reduction identified increased activity, connectivity and volume in the prefrontal cortex and hippocampus in stressed, anxious and healthy adults.15 Also, the amygdala demonstrated decreased activity and improved functional connectivity with the prefrontal cortex. Although yet to be studied in patients with chronic pain, these neuroplastic changes could potentially promote improved cortical dynamics in our patients.
I am excited to share this model of chronic stress and chronic pain and evidence-based applications of mindfulness to pain treatment in my upcoming course Mindfulness-Based Pain Treatment in Arlington, VA August 4 and 5, 2018 and in Seattle, WA November 3 and 4, 2018. Course participants will learn about mindfulness and pain research, practice mindful breathing, body scan and movement and expand their pain treatment tool box with practical strategies to improve pain treatment outcomes. Research examining the application of mindfulness in the treatment of patients at risk of opioid misuse will be included. I hope you will join me!
Vachon-Presseau E. Effects of stress on the corticolimbic system: implications for chronic pain. Prog Neuropsychopharmacol Biol Psychiatry. 2017; Oct 25. pii: S0278-5846(17)30598-5.
Arnsten AF. Stress signaling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 2009:10(6):410-422.
Zhang X, Tong G, Guanghao Y, et al. Stress-induced functional alterations in amygdala: implications for neuropsychiatric diseases. Front Neurosci. 2018 May 29;12:367.
Kim EJ, Pellman B, Kim JJ. Stress effects on the hippocampus: a critical review. Learn Mem. 2015;22(9):411-6.
Fritz HC, McAuley JH, Whittfeld K, et al. Chronic back pain is associated with decreased prefrontal and anterior insular gray matter: results from a population-based cohort study. J Pain. 2016;17(1):111-8.
Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. J Mol Psychiatry. 2013;1(1):9.
Vachon-Presseau E. Roy M, Martel MO, et al. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function. Brain. 2013;136(Pt 3):815-27.
Greco JA, Liberzon I. Neuroimaging of fear-associated learning. Neuropsychopharmacology. 2016;41(1):320-334.
Mansour AR, Farmer MA, Baliki. Chronic pain: role of learning and brain plasticity. Restor Neurol Neurosci. 2014;32(1):129.
Baliki MN, Apkarian AV. Nociception, pain, negative moods and behavior. Neuron. 2015;87(3):474-491.
Schmaal L, Veltman DJ, van Erp TG, et al. Subcortical brain alterations in major depressive disorder: findings from ENIGMA major depressive disorder working group. Mol Psychiatry. 2016;21(6):806-12.
Shin LM, Liberzon I. The neurocircuitry of fear, stress and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169-91.
Greenwald J, Shafritz KM. An integrative neuroscience framework for the treatment of chronic pain: from cellular alterations to behavior. Front Int Neurosci. 2018 May 23;12:18.
Seminowicz DA, Shpaner M, Keaser ML, et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain. 2013;14(2):1573-84.
Gotink RA, Meijboom R, Vernooij, et al. 8-week Mindfulness Based Stress Reduction induces brain changes similar to traditional long-term meditation practice – A systematic review. Brain Cogn. 2016;108:32-41.
The British author, John Donne, wrote, “No man is an island, entire of itself; every man is a piece of the continent.” In a similar idea, no neurological symptom is independent and isolated; every system has potential to impact the whole body. Neurogenic bladder should cue a clinician to check for neurogenic bowel and to assess the pelvic floor in order to get a complete map of what to address in treatment.
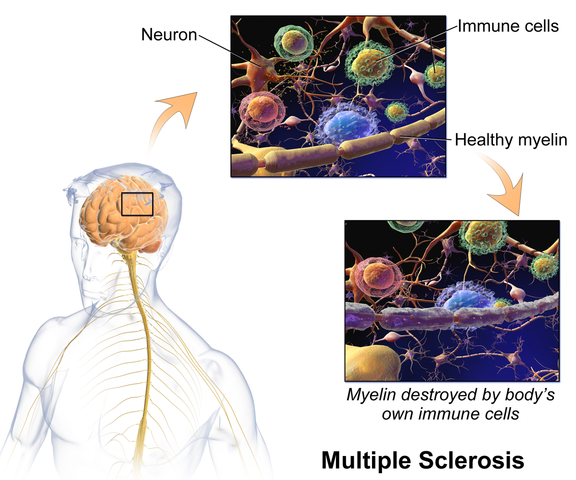 Martinez, Neshatian, & Khavari (2016) reviewed literature on neurogenic bowel dysfunction (NBD) and neurogenic bladder in patients with neurological conditions such as multiple sclerosis (MS). Constipation and fecal incontinence can coexist with NBD, and a multifactorial bowel regimen is vital to conservative management in patients with neurological disorders. Nonpharmacological, pharmacological, and surgical approaches were reviewed in the article. Specific results for MS were reported only for transanal irrigation (TAI) and biofeedback. In TAI, fluid is used to stimulate the bowel and clean out stool from the rectum. A study showed 53% of the 30 patients with MS demonstrated a 50% or better improvement in bowel symptoms with TAI. In anorectal biofeedback, operant conditioning retrains motor and sensory responses via exercises guided by manometry. With biofeedback, a study showed 38% of patients had a beneficial impact with the intervention. The list of treatment approaches not specifically researched for MS patients in this review includes: dietary modifications, perianal/anorectal stimulation, abdominal massage, suppositories, oral medications such as stool softeners or prokinetic agents, sacral neuromodulation, antegrade continence enema, and colostomy.
Martinez, Neshatian, & Khavari (2016) reviewed literature on neurogenic bowel dysfunction (NBD) and neurogenic bladder in patients with neurological conditions such as multiple sclerosis (MS). Constipation and fecal incontinence can coexist with NBD, and a multifactorial bowel regimen is vital to conservative management in patients with neurological disorders. Nonpharmacological, pharmacological, and surgical approaches were reviewed in the article. Specific results for MS were reported only for transanal irrigation (TAI) and biofeedback. In TAI, fluid is used to stimulate the bowel and clean out stool from the rectum. A study showed 53% of the 30 patients with MS demonstrated a 50% or better improvement in bowel symptoms with TAI. In anorectal biofeedback, operant conditioning retrains motor and sensory responses via exercises guided by manometry. With biofeedback, a study showed 38% of patients had a beneficial impact with the intervention. The list of treatment approaches not specifically researched for MS patients in this review includes: dietary modifications, perianal/anorectal stimulation, abdominal massage, suppositories, oral medications such as stool softeners or prokinetic agents, sacral neuromodulation, antegrade continence enema, and colostomy.
Miletta, Bogliatto, & Bacchio (2017) presented a case study about management of sexual dysfunction, perineal pain, and elimination dysfunction in a 40 year old female with multiple sclerosis. She had been experiencing perineal pain for 5 months and had chronic MS symptoms of lower anourogenital dysfunction, including bladder retention and obstructed defecation syndrome. Physical therapy treatment included pelvic floor muscle training (primarily decreasing overactivity of pelvic muscles in this case), perineal massage, biofeedback, postural correction, global relaxation techniques, and a home self-training program. After 5 months of physical therapy, the woman had improved pelvic floor muscle contraction strength, resolution of pelvic floor muscle overactivity, increased sexual satisfaction (according to the Female Sexual Function Index score), a visual analog scale improvement of vulvar and perineal pain by 4 points, normalization of obstructed defecation syndrome, and decreased bladder retention symptoms. The authors concluded the variety of symptoms in MS require a multimodal approach for treatment, considering all the motor, autonomic, and cognitive impairments as well as side effects of medications that try to improve those symptoms. The quality of life of women with MS has potential to be improved significantly if pelvic floor disorders related to MS are addressed appropriately.
Ultimately, treating urinary dysfunction but avoiding bowel dysfunction does neurological patients a disservice. Systems are intertwined in a series of cause and effects throughout the body. The “Neurologic Conditions and the Pelvic Floor” course can expand your knowledge and understanding of how the symptoms of conditions such as multiple sclerosis can impact pelvic health and how we can better address the whole patient for optimal outcomes.
Martinez, L., Neshatian, L., & Khavari, R. (2016). Neurogenic Bowel Dysfunction in Patients with Neurogenic Bladder. Current Bladder Dysfunction Reports, 11(4), 334–340. http://doi.org/10.1007/s11884-016-0390-3
Miletta, M., Bogliatto, F., & Bacchio, L. (2017). Multidisciplinary Management of Sexual Dysfunction, Perineal Pain, and Elimination Dysfunction in a Woman with Multiple Sclerosis. International Journal of MS Care, 19(1), 25–28. http://doi.org/10.7224/1537-2073.2015-082
Tamara Rial, PhD, CSPS, co-founder and developer of Low Pressure Fitness will be presenting the first edition of “Low Pressure Fitness and abdominal massage for pelvic care” in Princeton, New Jersey in July, 2018. Tamara is internationally recognized for her work with hypopressive exercise and Low Pressure Fitness. In this article she presents the novel topic of hypopressives as a complementary pelvic floor muscle training tool for incontinence after prostate cancer surgery.
Urinary Incontinence is the most common side effect men suffer after prostate cancer surgery along with erectile dysfunction. Although it is not life threatening, urinary incontinence definitely has a negative impact on the patient’s quality of life Sountoulides et al., 2013. Beyond the frustration and embarrassment associated with pelvic floor dysfunction, many patients describe it as depressing, disheartening and devastating.
The first line of conservative treatment - and most often recommended - is pelvic floor muscle training Andersen et al., 2015. Over the past few years, some researchers have also recommended alternative exercise programs with a holistic approach such as Pilates and hypopressives to improve the patient’s quality of life and urinary incontinence symptoms (Santa Mina et al., 2015). These alternative pelvic floor muscle training programs draw upon the connection between the pelvic floor, it’s synergistic muscles (abdominal, pelvic, lumbar) and their interrelated role in posture and breathing Hodges, 2007; Sapsford, 2004; Madill and McLean, 2008; Talasz et al., 2010. Among these complementary exercise programs, hypopressives have gained increasing attention for the recovery of post-prostatectomy urinary incontinence Santa Mina et al., 2015; Mallol-Badellino, et al. 2015.
What is known about hypopressives for post-prostatectomy incontinence?
Although hypopressive exercise has become popular for women, some researchers, clinicians and practitioners have begun to apply these exercises for specific male issues such as urinary incontinence following a prostatectomy. Recently, a case-study I co-authored about an adapted program of hypopressive exercise for urinary incontinence following a radical prostatectomy surgery was published in the Journal of the Spanish physiotherapy association Chulvi-Medrano & Rial, 2018. We describe the case of a 46-year-old male with severe stress urinary incontinence six months after surgery. We used a pelvic floor exercise program consisting of hypopressive exercises as described in the Low Pressure Fitness level 1 practical manual Rial & Pinsach, 2017 combined with contraction of the pelvic floor muscles. Satisfactory results were obtained after the rehabilitation protocol as evidenced by a reduction from 3 daily pads to none. Of note, clinical trails have demonstrated the benefits of initiating a rehabilitation program to strengthen the pelvic floor as soon as possible after prostatectomy. Previously, I’ve studied hypopressive exercise for female urinary incontinence Rial et al., 2015 and for the improvement of female athletes pelvic floor function Álvarez et al., 2016. However, this was the first time we’ve studied hypopressives in the context of male urinary leakage.
In the same light, other researchers have also included hypopressives in their pelvic floor training protocol for post-prostatectomy urinary incontinence. For example, Serda et al (2010) and Mallol-Badellino (2015) used protocols that combined pelvic floor contractions with postural re-education and hypopressives. Both studies found improvements in the severity of involuntary leakages and improvements in the patients’ quality of life. Similar results are also described in the clinical case by Scarpelini et al. (2014) who used hypopressives and psoas stretching exercises to reduce urinary incontinence after prostatectomy.
But how do hypopressives work?
The hypothesis underlying the use of hypopressives as a complementary pelvic floor and core exercise program is that it retrains the core system with specific postural and breathing strategies while reducing pressure on the pelvic organs and structures. The most striking part of hypopressives breathing technique is the abdominal vacuum. This breathing maneuver involves a low pulmonary volume exhale-hold technique followed by a rib-cage expansion involving the activation of the inspiratory muscles. The rib-cage expansion during the breath-holding phase leads to a noticeable draw-in of the abdominal wall and simultaneously to the rise of the thoracic diaphragm. Recent observational studies have shown how the hypopressive technique was able to elevate the pelvic viscera and to activate the pelvic floor and deep core muscles in women trained with hypopressives Navarro et al., 2017. From an historical point of view, this characteristic breathing maneuver was first described and practiced as a yoga pranayama called Uddiyanha Bandha Omkar & Vishwas, 2009.
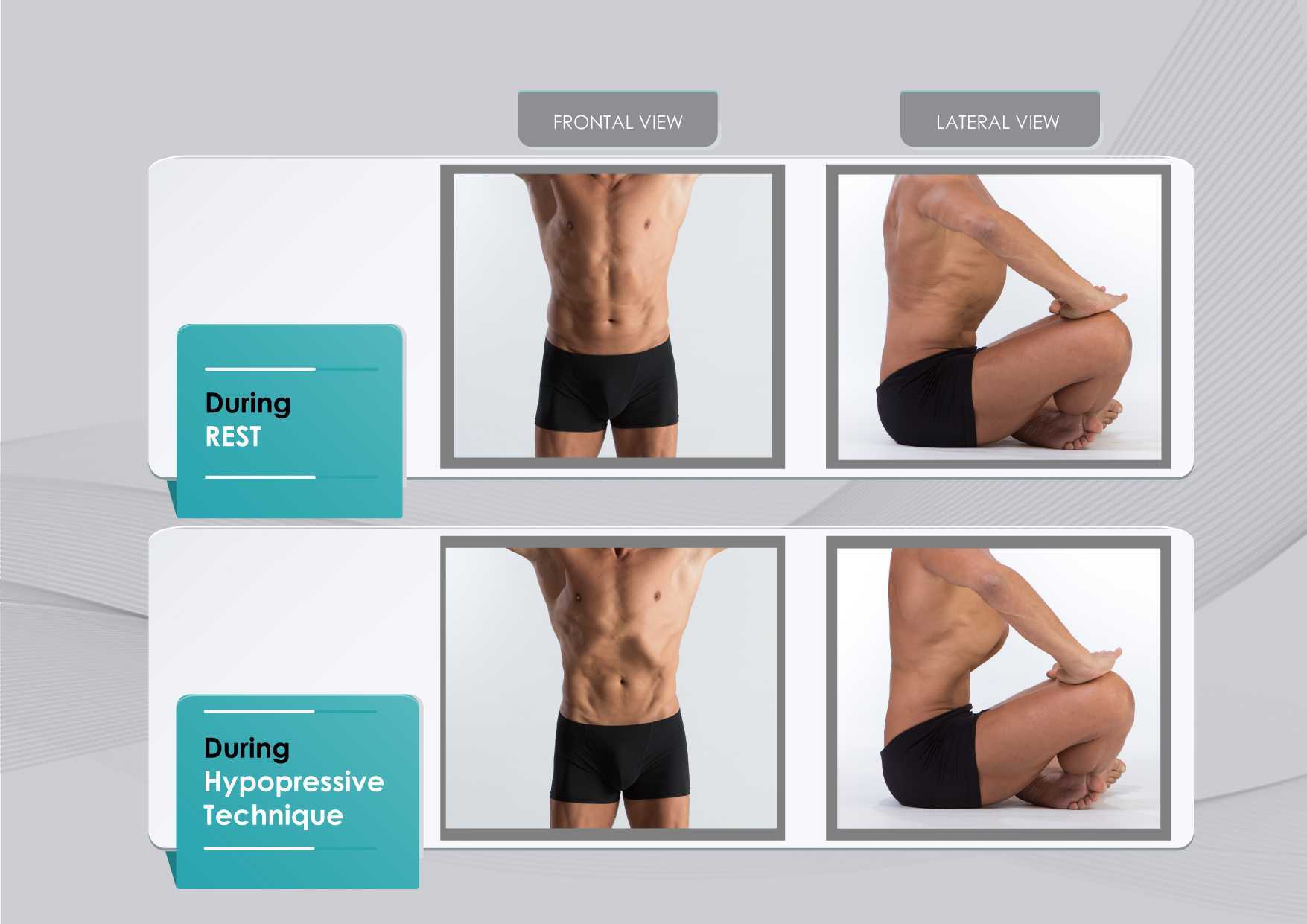
Figure 1 shows the anatomical behavior of the rib cage and the abdominal wall when performing the hypopressive breathing maneuver, which should not be confused with an abdominal hollowing, or a bracing maneuver. Anatomical observation of the thoracic and abdominal behavior during the breathing maneuver of the hypopressive exercise. Figure elaborated by the author.
In addition to breath control, the hypopressive technique involves a series of static and dynamic poses which operate on the hypothesis of training the stabilizing muscles of the spine, such as the core and pelvic muscles. In this sense, hypopressives are not exclusively a breathing technique, but rather they are an integrated whole-body technique. The practice of hypopressives involves body control, body awareness, postural correction and mindfulness throughout its different poses and postural techniques. The introduction of holistic exercise programs to train the synergist pelvic floor muscles and breathing patterns can be viewed as complementary tools for the restoration of a patient’s body awareness and functionality.
Another hypothesis of the effects of the hypopressive-breathing in the pelvic floor is the ability to move the pelvic viscera cranially as a consequence of the ribcage opening up after the breath-hold. This vacuum lifts the diaphragm and consequently creates an upward tension on the transversalis fascia, the peritoneum and other related fascial structures. In addition to the diaphragmatic suction effect, a correct alignment of the rib cage and pelvis during the exercise contributes to an improved suspension and position of the viscera in the pelvis. The mobility achieved with the breathing and its body sensations may be one of the reasons why hypopressives have also been recommended as a proprioceptive facilitator for those with low ability to “find their pelvic floor” Latorre et al., 2011.
It’s crucial to highlight that a complete surgical resection of the prostate will cause - in most of the cases - post-operative fibrosis and neurovascular damage Hoy-Land et al., 2014. Both, the neurovascular and musculoskeletal injuries are contributing factors for urinary incontinence post-prostatectomy. Subsequently, exercises focusing on increasing local vascular irrigation and re-activating the damaged musculature have been highlighted as the main goals to help patients recover continence. In this sense, breathing movements, fascia manipulation and decreased pelvic pressure can result in increased vascular supply. A previous study has shown an improvement in venous return of the femoral artery during the hypopressive-breathing maneuver Thyl et al., 2009. Collectively, all these factors may favor microcirculation in the pelvic area. Finally, the muscle activation of the pelvic floor and core muscles observed during the practice of hypopressives (Ithamar et al., 2017) and the changes of puborectalis and iliococcygeus muscles after an intensive pelvic floor muscle training (Dierick et al., 2018) are other factors that could have impact on urge incontinence, stress incontinence and overflow incontinence symptoms common after prostatectomy surgeries.
To date, the results from these investigations and clinical reports open new complementary pelvic floor training strategies for the treatment of post-prostatectomy incontinence. Hypopressives and pelvic floor muscle exercises are non-invasive, don’t require expensive material, and provide an exercise-based approach as part of a healthy lifestyle. However, qualified instruction, technique-driven progression and adherence to the intervention are critical components of any pelvic floor and hypopressive training protocol.
Álvarez M, Rial T, Chulvi-Medrano I, García-Soidán JL, Cortell JM. 2016. Can an eight-week program based on the hypopressive technique produce changes in pelvic floor function and body composition in female rugby players? Retos nuevas Tendencias en Educación Física, Deporte y Recreación, 30(2): 26-29.
Anderson CA, Omar MI, Campbell SE, Hunter KF, Cody JD, Glazener CM. 2015. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev, 1:CD001843.
Chulvi-Medrano I, Rial T. 2018. A case study of hypopressive exercise adapted for urinary incontinence following radical prostactetomy surgery. Fisioterapia, 40, 101-4. Doi: DOI: 10.1016/j.ft.2018.01.004
Dierick F, Galrsova E, Laura C, Buisseret F, Bouché FB, Martin L. 2018. Clinical and MRI changes of puborectalis and iliococcygeus after a short period of intensive pelvic floor muscles training with or without instrumentation. European Journal of Applied Physiology, doi:10.1007/s00421-018-3899-7
Ithamar, L., de Moura Filho, A.G., Benedetti-Rodrigues, M.A., Duque-Cortez, K.C., Machado, V.G., de Paiva-Lima, C.R.O., et al. 2017. Abdominal and pelvic floor electromyographic analysis during abdominal hypopressive gymnastics. J. Bodywork. Mov. Ther. doi: 10.1016/j.jbmt.2017.06.011.
Latorre G, Seleme M, Resende AP, Stüpp L, Berghmans B. Hypopressive gymnastics: evidences for an alternative training for women with local proprioceptive deficit of the pelvic floor muscles. Fisioterapia Brasil 2011; 12(6): 463-6.
Hodges P. 2007. Postural and respiratory functions of the pelvic floor muscles. Neurourol Urodyn, 26(3): 362-371.
Hoyland K, Vasdev N, Abrof A, Boustead G. 2014. Post-radical prostatectomy incontinence: etiology and prevention. Rev Urol. 16(4), 181-8.
Madill, S., McLean, L. 2008. Quantification of abdominal and pelvic floor muscle synergies in response to voluntary pelvic floor muscle ontractions. J. Electromyogr. Kinesiol. 18, 955-64. doi: 10.1016/j.jelekin.2007.05.001.
Mallol-Badellino J., et al. 2015. Resultados en la calidad de vida y la severidad de la incontinencia urinaria en varones prostatectomizados por neoplasia de próstata. Rehabilitación, 49(4); 210-215.
Navarro, B., Torres, M., Arranz, B. Sánchez, O. 2017. Muscle response during a hypopressive exercise after pelvic floor physiotherapy: Assessment with transabdominal ultrasound. Fisioterapia. 39, 187-194. doi:10.1016/j.ft.2017.04.003.
Omkar, S., Vishwas, B. 2009. Yoga techniques as a means of core stability training. J. Bodywork Mov. Thep. 13, 98-103. doi: 10.1016/j.jbmt.2007.10.004.
Rial T, Chulvi-Medrano I, Cortell-Tormo JM, Álvarez M. 2015. Can an exercise program based on hypopressive technique improve the impact of urinary incontinence on women´s quality of life? Suelo Pélvico, 11:27-32.
Rial, T., Pinsach, P. 2017. Low Pressure Fitness practical manual level 1. International Hypopressive and Physical Therapy Institute, Vigo.
Santa Mina D, Au D, Alibhai S, Jamnicky L, Faghani N, Hilton W, Stefanky L, et al. 2015. A pilot randomized trial of conventional versus advanced pelvic floor exercises on treat urinary incontinence after radical prostatectomy: a study protocol. BMC Urology, 15. DOI 10.1186/s12894-015-0088-4
Sapsford R. 2004. Rehabilitation of pelvic floor muscles utilizing trunk stabilization. Man Ther, 9(1): 3-12.
Serdá B, Vesa, A. del Valle, y Monreal P. 2010. La incontinencia urinaria en el cáncer de próstata: diseño de un programa de rehabilitación. Actas Urológicas Españolas, 34(6): 522-30.
Scarpelini P, Andressa Oliveira F, Gabriela Cabrinha S, Cinira H. 2014. Protocolo de ginástica hipopressiva no tratamento da incontinência urinária pós-prostatectomia: relato de caso. UNILUS Ensino e Pesquisa, 11(23): 90-95
Talasz, H., Kofler, M., Kalchschmid, E., Pretterklieber, M., Lechleitner, M. 2010. Breathing with the pelvic floor? Correlation of pelvic floor muscle function and expiratory flows in healthy young nulliparous women. Int. Urogynecol. J. 21, 475-81. doi: 10.1007/s00192-009-1060-1.
Thyl S., Aude P, Caufriez M, Balestra C. 2009. Incidence de l'aspiration diaphragmatique associée à une apnée expiratoire sur la circulation de retour veineuse fémorale: étude par échographie-doppler. Kinésithérapie scientifique, 502; 27-30.
Exciting news! Carolyn McManus, Herman & Wallace instructor of Mindfulness-Based Pain Treatment, will be a presenter in programming at the International Association for the Study of Pain (IASP) World Congress on Pain in to be held in Boston, September 11 - 16. This conference brings together experts from around the globe practicing in multiple disciplines to share new developments in pain research, treatment and education. Participants from over 130 countries are expected to attend. The last time it was held in the U.S. was 2002, so it presents an especially exciting opportunity for those interested in pain to have this international program taking place in the U.S. Carolyn will present a workshop on mindfulness in a Satellite Symposia, Pain, Mind and Movement: Applying Science to the Clinic.
 Carolyn has been a leader in bringing mindfulness into healthcare throughout her over-30 year career. She recognized early on in her practice how stress amplified patients’ symptoms and, as she had seen the benefits of mindfulness in her own life, it was a natural progression to integrate mindful principles and practices into her patient care. An instructor for Herman and Wallace since 2014, she has developed two popular courses, Mindfulness-Based Pain Treatment and Mindfulness for Rehabilitation Professionals, enabling her to share her clinical and research experiences with her colleagues.
Carolyn has been a leader in bringing mindfulness into healthcare throughout her over-30 year career. She recognized early on in her practice how stress amplified patients’ symptoms and, as she had seen the benefits of mindfulness in her own life, it was a natural progression to integrate mindful principles and practices into her patient care. An instructor for Herman and Wallace since 2014, she has developed two popular courses, Mindfulness-Based Pain Treatment and Mindfulness for Rehabilitation Professionals, enabling her to share her clinical and research experiences with her colleagues.
For many patients, pain is not linearly related to tissue damage and interventions based on structural impairment alone are inadequate to provide full symptom relief. Mindfulness training can offer a key ingredient necessary for a patient to make additional progress in treatment. By learning therapeutic strategies to build body awareness and calm an over-active sympathetic nervous system, patients can mitigate or prevent stress-induced symptom escalation. They can learn to move with trust and confidence rather than fear and hesitation.
A growing body of research in mindfulness-based therapies demonstrates multiples benefits for patients suffering with pain conditions. Research suggests that mindfulness training can be helpful to women preparing for childbirth and patients suffering from fibromyalgia, pelvic pain, IBS and low back pain. In addition, for patients with anxiety, mindfulness training may contribute to reductions in anxiety and in adrenocorticopropic hormone and proinflammatory cytokine release in response to stress. Authors of this study conclude that these large reductions in stress biomarkers provide evidence that mindfulness training may enhance resilience to stress in patients with anxiety disorders.
In addition to her presentation at the IASP World Congress Satellite Symposia, Carolyn will be sharing a more in-depth examination and practice of mindfulness in her upcoming course Mindfulness-Based Pain Treatment, August 4 and 5 at Virginia Hospital Center, Arlington VA, and again November 3 and 4 at Pacific Medical Center in Seattle, WA. Please join an internationally-recognized expert for 2 days of innovative training in mindfulness that will both improve your patient outcomes and enhance your own well-being!
Duncan LG, Cohn MA, Chao MT, et al. Benefits of preparing for childbirth with mindfulness training: A randomized controlled trial. BMC Pregnancy Childbirth 2017 May 12;17(1):140.
Fox SD, Flynn E, Allen RH. Mindfulness meditation for women with chronic pelvic pain: a pilot study. J Reprod Med.2011;56(3-4):158-62.
Garland EL, Gaylord SA, Paisson O. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing and affective processing of pain sensations. J Behav Med. 2012;35(6):591-602.
Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized Clinical Trial. JAMA. 2016;315(12):1240-9.
Hoge EA, Bui E, Palitz SA, et al. The effect of mindfulness meditation training on biological acute stress responses in generalized anxiety disorder. Psychiatry Res. 2018;262:328-332.
Akinesia is a term typically used to describe the movement dysfunction observed in people with Parkinson disease. It is defined as a poverty of movement, an impairment or loss of the power to move, and a slowness in movement initiation. There is an observable loss of facial expression, loss of associated nonverbal communicative movements, loss of arm swing with gait, and overall small amplitude movements throughout all skeletal muscles in the body. The cause of this characteristic profile of movement is due to loss of dopamine production in the brain which causes a lack of cortical stimulation for movement.
 If the loss of dopamine production in the brain causes this poverty of movement in all skeletal muscles the body, how does the pelvic floor function in the person with Parkinson disease and what should the pelvic floor rehabilitation professional know about treating the pelvic floor in this population of patients?
If the loss of dopamine production in the brain causes this poverty of movement in all skeletal muscles the body, how does the pelvic floor function in the person with Parkinson disease and what should the pelvic floor rehabilitation professional know about treating the pelvic floor in this population of patients?
Let’s take a closer look referencing a very telling article about Parkinson disease and skeletal muscle function. In the Italian town of L’Aquila, a major devastating 6-point Richter scale earthquake occurred on April 6, 2009. 309 people died and there was destruction and collapse of many historical structures, some greater than 100 years old. The nearby movement disorder clinic had been following 31 Parkinson disease patients in the area, 17 of them higher functioning and the other 14 much lower functioning. In fact, of those 14, 10 of them were affected by severe freezing episodes with severe nighttime akinesia requiring assistance with bed mobility tasks, 1 was completely bedridden and the others with major fluctuations in motor performance. 13 of the 14 patients also had fluctuating cognitive functioning.
This devastating earthquake occurred at 3:30 am. All 14 of these patients were able to escape from their homes during or immediately following the event. Caregivers reported that in the majority of the cases, the person with Parkinson’s disease was the first one to be alerted to the earthquake, the first one to get out of the house, ability to alert relatives to run for safety, physically assisting relatives out of the collapsing buildings, and in some cases independently escaping down 1-2 flights of stairs.
Paradoxical kinesia is thought to be the reason for this all but sudden ability to move normally within the presence of an immediate threat to their life and lives of loved ones. Paradoxical kinesia is defined as “a sudden and brief period of mobility typically seen in response to emotional and physical stress in patient’s with advanced idiopathic Parkinson’s disease.” There are a few mechanisms hypothesized to play a role, such as, adrenaline, dopaminergic reserves activating the flight reaction, and compensatory nearby cerebellar circuitry.
There is no pathological evidence that in Parkinson disease there is any break in the continuity of the motor system. The neurologic pathways are all intact and the ability to produce muscle power is retained however requires a strong base of clinic knowledge of the disease to help these patients activate these intact motor pathways. I look forward to sharing the neurologic basis of these deficits in Parkinson disease and strategies in pelvic floor rehab to do just that!
Erica Vitek, a specialist in treating patients with neurologic dysfunction, is the author and instructor of Neurologic Conditions and Pelvic Floor Rehab, taking place September 14-16, 2018 in Grand Rapids, MI.
Bonanni, L., Thomas, A., Anzellotti, F., Monaco, D., Ciccocioppo, F., Varanese, S., Bifolchetti, S., D’Amico, M.C., Di Iorio, A. & Onofrj, M. (2010). Protracted benefit from paradoxical kinesia in typical and atypical parkinsonisms. Neurological sciences, 31(6), 751-756.
In a previous post on The Pelvic Rehab Report, Sagira Vora, PT, MPT, WCS, PRPC explored the impact that pelvic floor exercises can have on arousal and orgasm in women. Today we hear part two of the conversation, and learn what factors can impact a woman's ability to achieve orgasm.
 “An orgasm in the human female is a variable, transient peak sensation of intense pleasure, creating an altered state of consciousness, usually with an initiation accompanied by involuntary, rhythmic contractions of the pelvic striated circumvaginal musculature, often with concomitant uterine and anal contractions, and myotonia that resolves the sexually induced vasocongestion and myotonia, generally with an induction of well-being and contentment.”
“An orgasm in the human female is a variable, transient peak sensation of intense pleasure, creating an altered state of consciousness, usually with an initiation accompanied by involuntary, rhythmic contractions of the pelvic striated circumvaginal musculature, often with concomitant uterine and anal contractions, and myotonia that resolves the sexually induced vasocongestion and myotonia, generally with an induction of well-being and contentment.”
Wow, that sounds like paradise! The question is--how to get there? Many of our cohorts and many our female patients have not experienced this or orgasm happens for them rarely. Findings from surveys and clinical reports suggest that orgasm problems are the second most frequently reported sexual problems in women. Some of the reasons cited for lack of orgasm are orgasm importance, sexual desire, sexual self-esteem, and openness of sexual communication with partner by Kontula el. al. in 2016. Rowland found that most commonly-endorsed reasons were stress/anxiety, insufficient arousal, and lack of time during sex, body image, pain, inadequate lubrication.
One factor that comes up consistently, is the ability of women to focus on sexual stimuli. This point has been brought up by various studies and presented in different ways. Chambless talks about mindfulness training and improvements in orgasm ability noted equally in women who practiced mindfulness vs. women who engaged in Kegels and mindfulness. Rosenbaum and Padua note in their book, The Overactive Pelvic Floor, “women who do not have a low-tone pelvic floor and who seek to enhance sexual arousal and more frequent orgasms have not much to gain from pelvic floor muscle training. Actually, a relaxed pelvic floor and mindful attention to sexual stimuli and bodily sensations seem a more effective means of enhancing sexual arousal and orgasm.” Various studies specifically studying the effect of mindfulness training have demonstrated both improved arousal and orgasm ability in women who practiced mindfulness. Brotto and Basson found their treatment group, which consisted of 68 otherwise healthy women, who underwent mindful meditation, cognitive behavioral training and education, improved in sexual desire, sexual arousal, lubrication, sexual satisfaction, and overall sexual functioning.
Cognitive-behavioral therapy appears to play a significant role in improving sexual function in women. Meston et. al. notes, “cognitive behavioral therapy for anorgasmia focuses on promoting changes in attitudes and sexually relevant thoughts, decreasing anxiety, and increasing orgasmic ability and satisfaction. To date there are no pharmacological agents proven to be beneficial beyond placebo in enhancing orgasmic function in women.”
Alas, there are no magic pills to create the above described “state of altered consciousness,” allowing women a sense of “well-being and contentment.” However, mindfulness training and cognitive behavioral therapy are both accessible and attainable for women who want to improve their ability to enjoy this much desired state. Many Pelvic floor therapist incorporate cognitive behavioral and mindfulness approaches in their practice.
The studies above mention pain as one of the factors for inability to experience arousal and orgasm. Hucker and Mccabe even noted that their mindfulness treatment group demonstrated significant improvements in all domains of female sexual response except for sexual pain. Dealing with sexual pain is a daily battle pelvic floor therapist face each day. So, how do women with sexual pain dysfunction differ from women who are experiencing sexual dysfunction but not pain? Let’s explore this in our next blog…
Chambless DL, Sultan FE, Stern TE, O’Neill C, Garrison S. Jackson A. Effect of pubococcygeal exercise on coital orgasm in women. J Consult CLin Psychol. 1984; 52:114-8
Bratto LA, Basson R. Group mindfulness-based therapy significantly improves sexual desire in women Behav Res Ther. 2014 Jun; 57:43-5
Hucker A. Mccabe MP. Incorporating Mindfulness and Chat Groups Into an Online Cognitive Behavioral Therapy for Mixed Female Sexual Problems. J Sex Res. 2015;52(6):627-33
Kontula O., Mettienen A. Determinants of female sexual orgasms. Socioaffect Neurosci Psychol. 2016 Oct 25;6:31624. doi: 10.3402/snp.v6.31624. eCollection 2016
Meston CM1, Levin RJ, Sipski ML, Hull EM, Heiman JR. Women’s orgasm. Annu Rev Sex Res. 2004;15:173-257. Review
Rosenbaum, Talli Y., Padoa, Anna. The overactive Pelvic floor. 1st ed. 2016
Roland DL, Cempel LM, Tempel AR. Women’s attributions on why they have difficulty reaching orgasm. J. Marital Therapy. 2018 Jan 3:0
Kelly Feddema, PT, PRPC returns in a guest post on Pregnancy Associated Ligamentous Laxity. Kelly practices pelvic floor physical therapy in the Mayo Clinic Health System in Mankato, MN, and she became a Certified Pelvic Rehabilitation Practitioner in February of 2014. See her post on diastasis recti abdominis on the pelvic rehab report, and learn more about evaluating and treating pregnant patients by attending Care of the Pregnant Patient!
 Pregnancy associated ligamentous laxity is something that we, as therapists, are fairly well aware of and see the ramifications of quite often in the clinic. We know the female body is changing to allow the mother to prepare for the growth and birth of the tiny (or sometimes not so tiny) human she is carrying. We also know that the body continues to evolve after the birth to eventually return to a post-partum state of hormonal balance. Do we think much about what this ligamentous laxity can mean during the actual delivery? Does laxity predispose women to other obstetric injury?
Pregnancy associated ligamentous laxity is something that we, as therapists, are fairly well aware of and see the ramifications of quite often in the clinic. We know the female body is changing to allow the mother to prepare for the growth and birth of the tiny (or sometimes not so tiny) human she is carrying. We also know that the body continues to evolve after the birth to eventually return to a post-partum state of hormonal balance. Do we think much about what this ligamentous laxity can mean during the actual delivery? Does laxity predispose women to other obstetric injury?
A recent study in the International Urogynecology Journal assessed ligamentous laxity from the 36th week of pregnancy to the onset of labor by measuring the passive extension of the non-dominant index finger with a torque applied to the second metacarpal phalangeal joint. They collected the occurrence and classification of perineal tears in 272 out of 300 women who ended up with vaginal deliveries and looked for a predictive level of second metacarpophalangeal joint (MCP) laxity for obstetric anal sphincter injury (OASI). They concluded that the increased ligamentous laxity did seem associated with OASI occurrence which was opposite of their initial idea that more lax ligaments would be at less of a risk of OASI.
In another study from the same journal published in 2017, researchers studied if levator hiatus distension was associated with peripheral ligamentous laxity during pregnancy. This was a small study but they concluded that levator hiatus distension and ligamentous laxity were significantly associated during pregnancy. They did admit the relationship was weak and results would have to be confirmed with a larger study and more specific study methods. However, the likelihood of major levator trauma more than triples during the reproductive years from under 15% at age 20 to over 50% at age 40(University of Sydney) so it seems that these issues warrant continued study with the continued trend toward delayed child bearing in Western cultures.
Gachon, B., Desgranges, M., Fradet, L. et al. Int Urogynecol J (2018). https://doi.org/10.1007/s00192-018-3598-2
Gachon, B., Fritel, X., Fradet, L. et al. Int Urogynecol J (2017) 28: 1223. https://doi.org/10.1007/s00192-016-3252-9
University of Sydney. "Levator Trauma" sydney.edu.au. Accessed 25 April 2018.

















































